Solid-state Batteries
Solid-state batteries are considered as a next-generation battery technology with many potential improvements over the current state-of-the-art Li-ion in terms of safety, power and energy density. Enabling this technology relies on the discovery and application of solid electrolytes (see also Solid State Ionics section) that replace the currently used liquid solutions. The all-solid nature of the resulting cells necessitates very careful control of the microstructure and pressure. The solid-state concept can also be combined with other post-Li technologies such as Li-S, Na(-ion), F-ion which are all studied in our group.
Recent highlights of the group’s work in this area include:
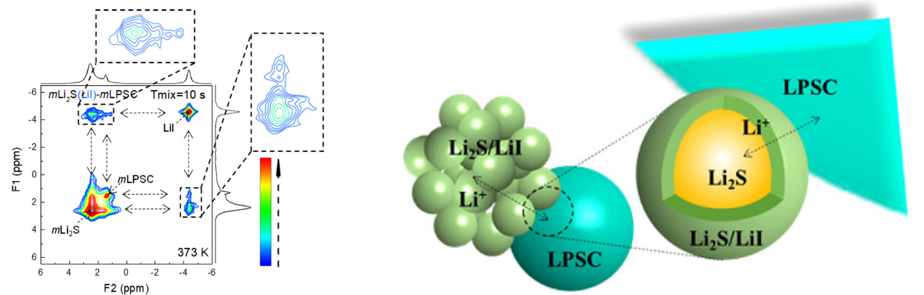
-Ming’s work on improving the interfacial contact between solid electrolytes and Li2S cathode particles by coating with LiI, published in Nature Communications 1–3. Through exchange-NMR we could elucidate the chemical nature of the solid electrolyte-cathode interface allowing us to optimize of the solid-state cell and achieve efficient sulphur redox without solid electrolyte decomposition.
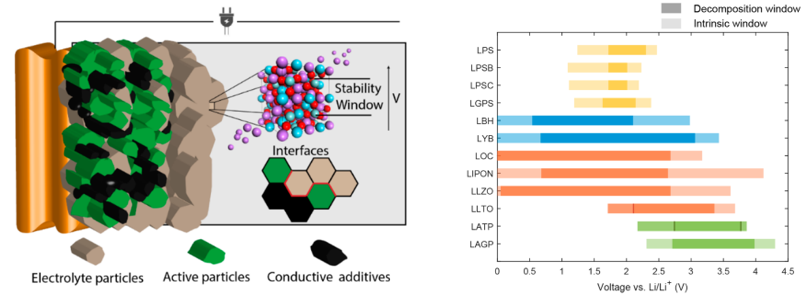
-Tammo and Viola’s nuanced description of voltage‑dependent decomposition of solid electrolytes, published in Nature Materials 4,5. Through combination of ab initio calculations and electrochemical measurements we showed that most solid electrolytes exhibit some flexibility with regards to their lithium content, which becomes critical to consider when considering their decomposition against solid-state battery electrodes.
References
(1) Liu, M.; Wang, C.; Zhao, C.; van der Maas, E.; Lin, K.; Arszelewska, V. A.; Li, B.; Ganapathy, S.; Wagemaker, M. Quantification of the Li-Ion Diffusion over an Interface Coating in All-Solid-State Batteries via NMR Measurements. Nat. Commun. 2021, 12 (1), 1–10. doi.org/10.1038/s41467-021-26190-2.
(2) Yu, C.; Ganapathy, S.; De Klerk, N. J. J.; Roslon, I.; Van Eck, E. R. H.; Kentgens, A. P. M.; Wagemaker, M. Unravelling Li-Ion Transport from Picoseconds to Seconds: Bulk versus Interfaces in an Argyrodite Li6PS5Cl-Li2S All-Solid-State Li-Ion Battery. J. Am. Chem. Soc. 2016, 138 (35), 11192–11201. doi.org/10.1021/jacs.6b05066.
(3) Yu, C.; Ganapathy, S.; Eck, E. R. H. van; Wang, H.; Basak, S.; Li, Z.; Wagemaker, M. Accessing the Bottleneck in All-Solid State Batteries, Lithium-Ion Transport over the Solid-Electrolyte-Electrode Interface. Nat. Commun. 2017, 8 (1), 1086. doi.org/10.1038/s41467-017-01187-y.
(4) Schwietert, T. K.; Arszelewska, V. A.; Wang, C.; Yu, C.; Vasileiadis, A.; de Klerk, N. J. J.; Hageman, J.; Hupfer, T.; Kerkamm, I.; Xu, Y.; van der Maas, E.; Kelder, E. M.; Ganapathy, S.; Wagemaker, M. Clarifying the Relationship between Redox Activity and Electrochemical Stability in Solid Electrolytes. Nat. Mater. 2020. doi.org/10.1038/s41563-019-0576-0.
(5) Schwietert, T. K.; Vasileiadis, A.; Wagemaker, M. First-Principles Prediction of the Electrochemical Stability and Reaction Mechanisms of Solid-State Electrolytes. JACS Au 2021, 1 (9), 1488–1496. doi.org/10.1021/jacsau.1c00228.