Hydrogen Sensors
Hydrogen sensors and other applications of thin film metal hydrides
(Thin film) metal hydrides have traditionally been studied as materials to store hydrogen. Meanwhile, new applications have emerged as for example solid electrolytes, switchable mirrors and hydrogen sensors. My work focusses on the last one. The working principle of these metal hydride hydrogen sensors is based on the fact that the optical properties change when metal hydrides partly hydrogenate when they are exposed to a hydrogen atmosphere. Compared to conventional ways of detecting hydrogen optical fiber hydrogen sensors are inherently safe, do not require the presence of oxygen, and can be made small and inexpensive.
In this research, we often make use of nanoconfinement effects: the propensity of materials to have different properties when materials are confined as e.g. in two-dimensional thin films. Recently, we discovered a material that can hysteresis-free sense hydrogen both at room and elevated pressures over 7 order of magnitude in partial hydrogen pressure with response times shorter than a second. This research is performed in close collaboration with Herman Schreuders and Prof. Bernard Dam from the Chemical Engineering department of Delft University of Technology.
More information about our research on hydrogen sensing materials can be found in a recent article in ‘The Physicus’, the magazine of the study association for Applied Physics:
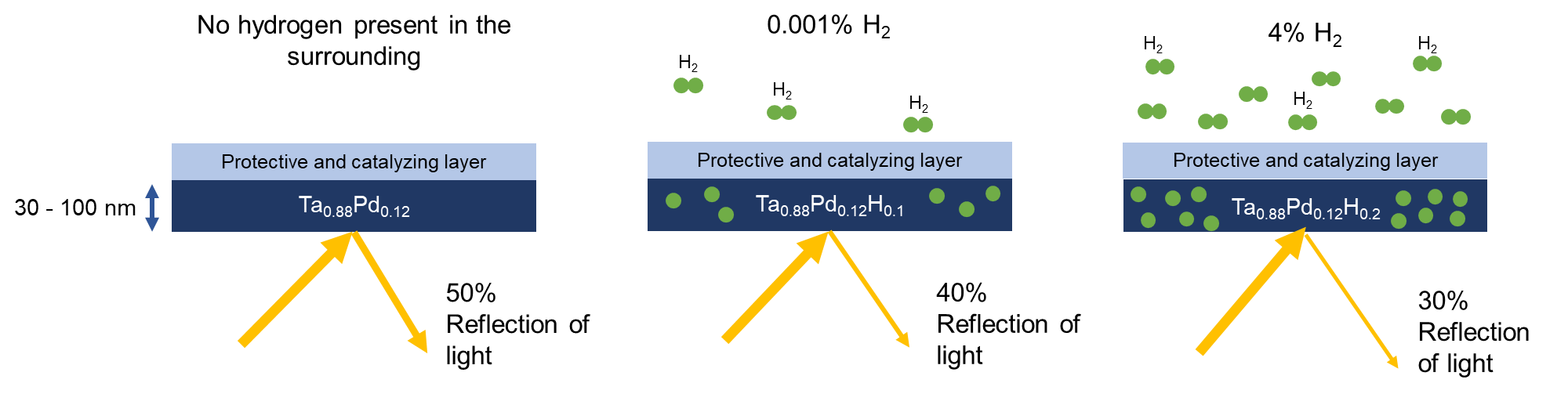
Figure 1: Schematic illustration of the working principle of optical hydrogen sensors. In the proximity of hydrogen, a metal hydride (in this case an alloy of tantalum (Ta) and palladium (Pd)) absorbs hydrogen). The higher the partial hydrogen pressure/ hydrogen concentration in the area, the more hydrogen the material absorbs. When the material absorbs hydrogen, its optical properties change. By for example measuring the amount of light reflected by the material one can determine the hydrogen pressure or concentration.
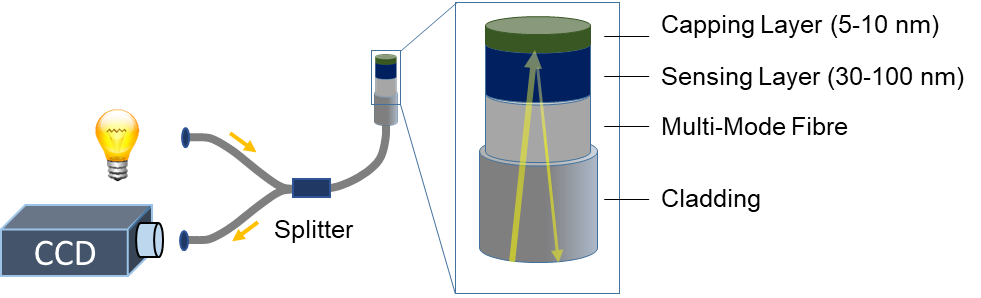
Figure 2. Schematic illustration of a micro-mirror optical hydrogen sensor. Light is coupled into a fiber in which it is transported to the tip of the optical fiber sensor. This tip partially reflects the light, and the reflected light is then passed back through the fibre and the splitter. The reflected light is subsequently measured by a photodetector as, for example, a charge-coupled device (CCD). When the hydrogen pressure changes, the hydrogenation of the sensing layer (30–100 nm) changes, resulting in a change of the optical properties and thus the amount of light reflected by the layer stack and detected by a photodetector. A capping layer is used to prevent oxidation of the sensing layer and to catalyze the hydrogen adsorption.

References
[1] Bannenberg, L. J., Schreuders, H., & Dam, B. (2021). Tantalum‐Palladium: Hysteresis‐Free Optical Hydrogen Sensor Over 7 Orders of Magnitude in Pressure with Sub‐Second Response. Advanced Functional Materials, 31(16), 2010483.
[2] Bannenberg, L. J., Boelsma, C., Asano, K., Schreuders, H., & Dam, B. (2020). Metal Hydride Based Optical Hydrogen Sensors. Journal of the Physical Society of Japan, 89(5), 051003.
[3] Bannenberg, L. J., Boshuizen, B., Nugroho, F.A.A., Schreuders, H. (2021) Hydrogenation Kinetics of Metal Hydride Catalytic Layers. ACS Appl. Mater. Interfaces, 13 (44), 52530–52541.
[4] Bannenberg, L.J. and Schreuders, H. (2020). (Optical) thin-film hydrogen sensing material based on tantalum or other group V element alloy. Patent Application No. NL 2026815.
[5] Bannenberg, L. J., Schreuders, H., Kim, H., Sakaki, K., Hayashi, S., Ikeda, K., Asono, K., Dam, B. (2021). Suppression of the Phase Coexistence of the fcc–fct Transition in Hafnium-Hydride Thin Films. The journal of physical chemistry letters, 12, 10969-10974.