Materials for Solid Oxide Cells
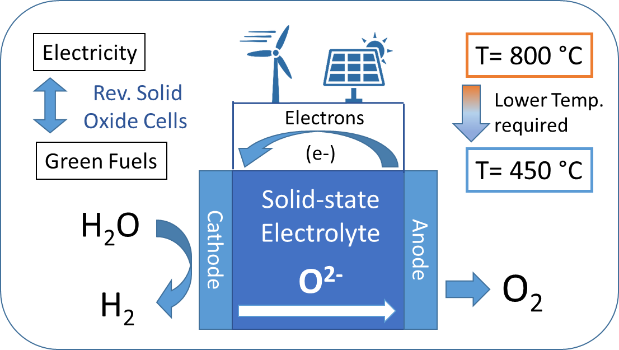
Reversible Solid Oxide Cells (RSOC) are promising chemical reactors for the decarbonized and electrified chemical plants of the future and a promising and flexible approach to the corresponding e-Refinery (link) concept.
When in electrolysis mode, these efficient electrochemical reactors can promote chemical reactions that would not otherwise occur spontaneously, as for example the splitting of water molecules to produce hydrogen gas (H2)[11] as a green fuel or the conversion of CO2 into useful chemical commodities, such as carbon monoxide (CO)[12] and methane (CH4). If powered by electricity from renewables, it provides a means to store electrical energy from intermittent sources into the chemical bonds of reactive molecules. By the same token, when used in the fuel cell mode, reversible solid oxide cells act as generators, and can convert reactive fuel molecules (e.g. H2 or CH4) and oxygen (O2) from air directly into electricity, with an efficiency higher than combustion engines.
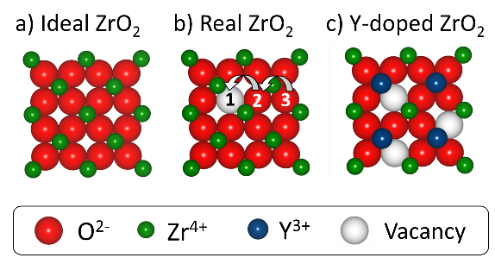
Their working principle relies on the efficient separation between the flow of electrons, as an external electric current, from the transport of O2- ions within the device (Fig 2). The standard solid-state electrolyte, yttrium-stabilized zirconium oxide (YSZ), can only transport O2- ions at high enough rates above 800 oC, which sets demanding requirements and high costs for its operation.[14]
Higher rates for O2- transport can be achieved by artificially introducing foreign metal ions (dopants) of similar size in a host structure, a process called doping (Fig 3c). Dopant ions with a lower electric charge than host metal ions require a lower number of O2- ions to achieve charge neutrality, such as the substitution of Zr4+ ions by yttrium ions Y3+ in Y-doped ZrO2 (YSZ).[14] Consequently, additional vacant oxygen sites are generated in a similar number as the amount of dopant ions (Fig. 3c). The resulting larger number of pathways for oxygen transport contributes to a higher ionic conductivity, thereby enhancing the solid electrolyte’s performance.
For a widespread use of solid oxide cells, higher conductivities are required between 450 oC and 600 oC,[14] but a breakthrough in conductivity is less likely to emerge from fine tuning of well-studied solid electrolytes, such as YSZ. Therefore, alternative classes of oxide ion conductors are required to realize this vision.
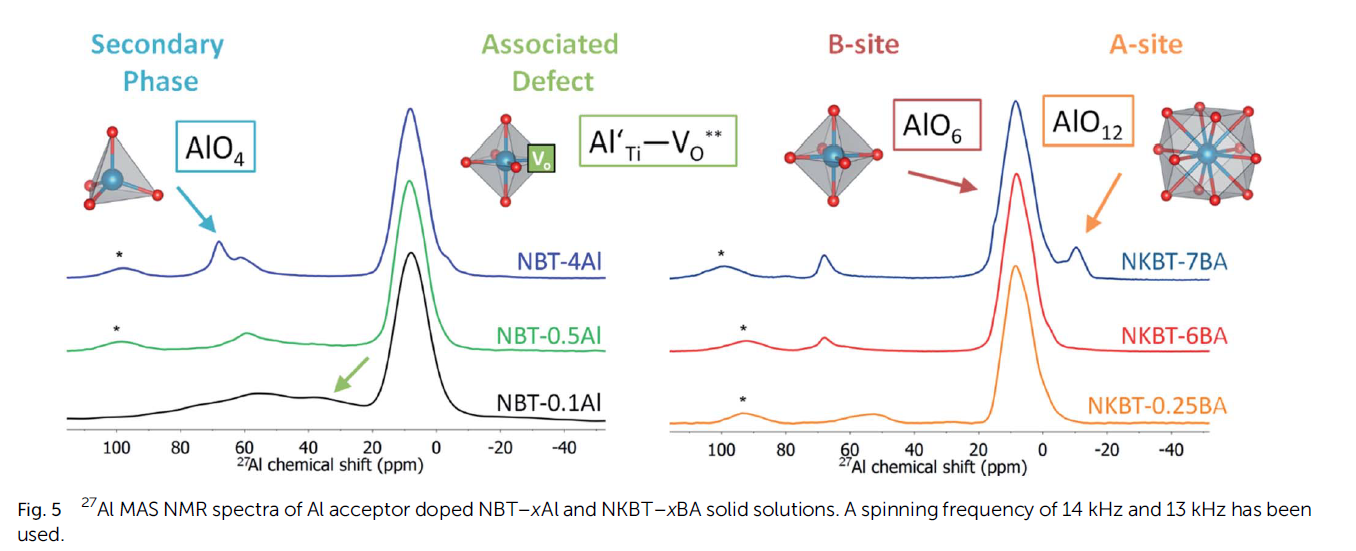
Our group focuses on combining the synthesis of novel oxides with a judicial choice of dopants, to understand under. In particular, a combination of neutron diffraction and nuclear magnetic resonance spectroscopy offers an ideal tool to probe the light O2- oxide ions in metal oxide lattices. For example, in a recent publication we investigated the role of Al3+ dopants on perovskite oxides as alternative oxide ion conductors (link reference).
Moreover, the use of ex situ studies can help us understand how the material changes when exposed to the reactive gas mixtures to which solid electrolytes must withstand in reversible solid oxide cell environments. In addition to that, in situ investigations help to shed light on the influence of dopants on the material’s structure and its dynamics in conditions similar to end device’s operation. This information is crucial for the design of enhanced mechanism for O2- conduction, which may enable the widespread use of Reversible Solid Oxide Cells in the intermediate temperature regime.