Research
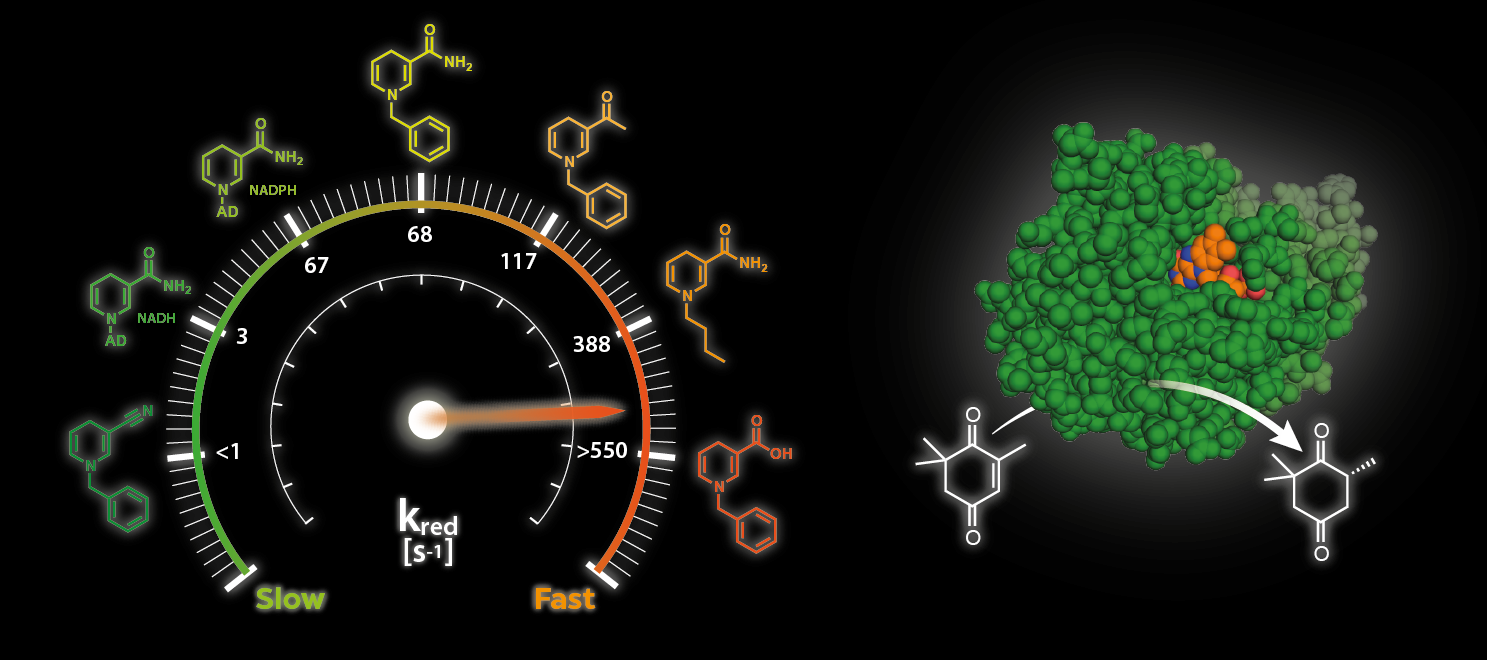
Our research interests focus on exploring the synthetic potential of Nature's catalysts, enzymes, that provide exquisite selectivity to access a range of products. Research lines include enzyme discovery and characterisation, designing nicotinamide coenzyme biomimetics (NCBs) for oxidoreductases, developing non-natural enzymatic reactions and multi-enzymatic cascades for applications in organic synthesis.
Research projects include:
- Engineering biomimetic cofactors (nicotinamide, flavin and S-adenosyl-L-methionine)
- Exploring the synthetic potential of reductases and flavoprotein monooxygenases
- Developing enzymatic cascades
- Enzyme discovery and characterisation of reductases (see Zero Emission Biotechnology Programme)
- Investigating light-driven sustainable biocatalysis H2020-MSCA-ITN-EJD PhotoBioCat
Selected work on cofactor biomimetics:
- Chem. Commun. 2022, 58, 10540, 10.1039/d2cc02411j
- Nat. Commun. 2022, 13, 5021, 10.1038/s41467-022-32727-w
- ChemBioChem 2022, 23, e202200293, 10.1002/cbic.202200293
- Catal. Sci. Technol. 2021, 11, 5077, 10.1039/d1cy00855b
- ChemCatChem 2020, 12, 1368–1375, 10.1002/cctc.201902044
- Curr. Opin. Biotechnol. 2019, 60, 63–71, 10.1016/j.copbio.2019.01.001 Review
- Catalysts 2019, 9, 207, 10.3390/catal9030207
- ACS Catal. 2019, 9, 1389–1395, 10.1021/acscatal.8b04500
- Angew. Chem. Int. Ed. 2018, 57, 13825–13828, 10.1002/anie.201804409
- Catalysts 2017, 7, 130, 10.3390/catal7050130
- Appl. Microbiol. Biotechnol., 2016, 100, 4773–4778, 10.1007/s00253-016-7500-1 Review
- J. Am. Chem. Soc. 2016, 138, 11089–11092, 10.1021/jacs.6b05625
- J. Am. Chem. Soc. 2016, 138, 1303–1309, 10.1021/jacs.5b12252
- ACS Catal. 2015, 5, 2961–2965, 10.1021/acscatal.5b00041
- ACS Catal. 2014, 4, 788–797, 10.1021/cs4011056 Review
- Org. Lett. 2013, 15, 180–183, 10.1021/ol303240a
Selected work on ene reductases:
- Asymmetric monoreduction of α,β-dicarbonyls to α-hydroxy carbonyls by ene reductases
- ACS Catal. 2024, 14, 15713–15720. 10.1021/acscatal.4c04676
- Biocatalytic reduction of alkenes in micro-aqueous organic solvent catalysed by an immobilised ene reductase.
- Catal. Sci. Technol. 2023, 13, 5530-5535. 10.1039/d3cy00541k
- Old Yellow Enzyme-catalysed asymmetric hydrogenation: linking family roots with improved catalysis.
- Catalysts 2017, 7, 130. 10.3390/catal7050130
- Better than Nature: nicotinamide biomimetics that outperform natural coenzymes.
- J. Am. Chem. Soc. 2016, 138, 1033-1039. 10.1021/jacs.5b12252
- Mimicking nature: synthetic nicotinamide cofactors for C=C bioreduction using enoate reductases.
- Org. Lett. 2013, 15, 180-183. 10.1021/ol303240a
Selected work on enzymatic cascades:
- Peroxygenase-catalyzed allylic oxidation unlocks telescoped synthesis of (1S,3R)-3-hydroxycyclohexanecarbonitrile.
- ACS Catal. 2024, 14, 2985, 10.1021/acscatal.4c00177
- Enantio-complementary synthesis of 2-substituted pyrrolidines and piperidines via transaminase-triggered cyclizations.
- JACS Au, 2023, 3, 1642, 10.1021/jacsau.3c00103
- Tunable production of (R)- or (S)-citronellal from geraniol via a bienzymatic cascade using a copper radical alcohol oxidase and Old Yellow Enzyme.
- ACS Catal. 2022, 12, 1111, 10.1021/acscatal.1c05334
- Synthesis of chiral amines via a bi-enzymatic cascade using an ene-reductase and amine dehydrogenase.
- ChemCatChem 2022, 14, e202101576, 10.1002/cctc.202101576
- Recent trends in synthetic enzymatic cascades promoted by alcohol dehydrogenases.
- Curr. Opin. Green Sustain. Chem. 2021, 32, 100548, 10.1016/j.cogsc.2021.100548 Review
- Asymmetric azidohydroxylation of styrene derivatives mediated by a biomimetic styrene monooxygenase enzymatic cascade.
- Catal. Sci. Technol. 2021, 11, 5077, 10.1039/D1CY00855B
Selected work on flavoprotein monooxygenases:
- Hybrid catalysis for enantioselective Baeyer-Villiger oxidation and stereoselective epoxidation: a Cp*Ir complex to fuel FMN and FAD reduction for flavoprotein monooxygenase modules. React. Chem. Eng. 2023, 8, 3117-3123. 10.1039/d3re00411b
- Flavoprotein monooxygenases: versatile biocatalysts. Biotechnol. Adv. 2021, 51, 107712. 10.1016/j.biotechadv.2021.107712
- Asymmetric azidohydroxylation of styrene derivatives mediated by a biomimetic styrene monooxygenase enzymatic cascade. Catal. Sci. Technol. 2021, 11, 5077-5085. 10.1039/d1cy00855b
- Flavoprotein monooxygenases and halogenases, book chapter 7 in Flavin‐Based Catalysis: Principles and Applications, 2021, pp. 169-199. 10.1002/9783527830138.ch7
- Divorce in the two-component BVMO family: the single oxygenase for enantioselective chemo-enzymatic Baeyer–Villiger oxidations. Org. Biomol. Chem. 2021, 19, 3441-3450. 10.1039/D1OB00015B
- Straightforward regeneration of reduced flavin adenine dinucleotide required for enzymatic tryptophan halogenation. ACS Catal. 2019, 9, 1389-1395. 10.1021/acscatal.8b04500
- Nonenzymatic regeneration of styrene monooxygenase for catalysis. ACS Catal. 2015, 5, 2961-2965. 10.1021/acscatal.5b00041
Selected work on light-driven biocatalysis:
- Light-driven NADPH cofactor recycling by photosystem I for biocatalytic reactions. ChemCatChem 2023, 15, e202300821. 10.1002/cctc.202300821
- Exciting enzymes: current state and future perspective of photobiocatalysis. ChemPhotoChem 2023, 7, e202200325. 10.1002/cptc.202200325
- Biocatalytic C=C bond reduction through carbon nanodot-sensitized regeneration of NADH analogues. Angew. Chem. Int. Ed. 2018, 57, 13825-13828. 10.1002/anie.201804409