Electrolysis of Green Hydrogen
Research Themes: Energy, High tech, Chemistry, bio- & process technology, Materials

A TRL is a measure to indicate the matureness of a developing technology. When an innovative idea is discovered it is often not directly suitable for application. Usually such novel idea is subjected to further experimentation, testing and prototyping before it can be implemented. The image below shows how to read TRL’s to categorise the innovative ideas.
Summary of the project
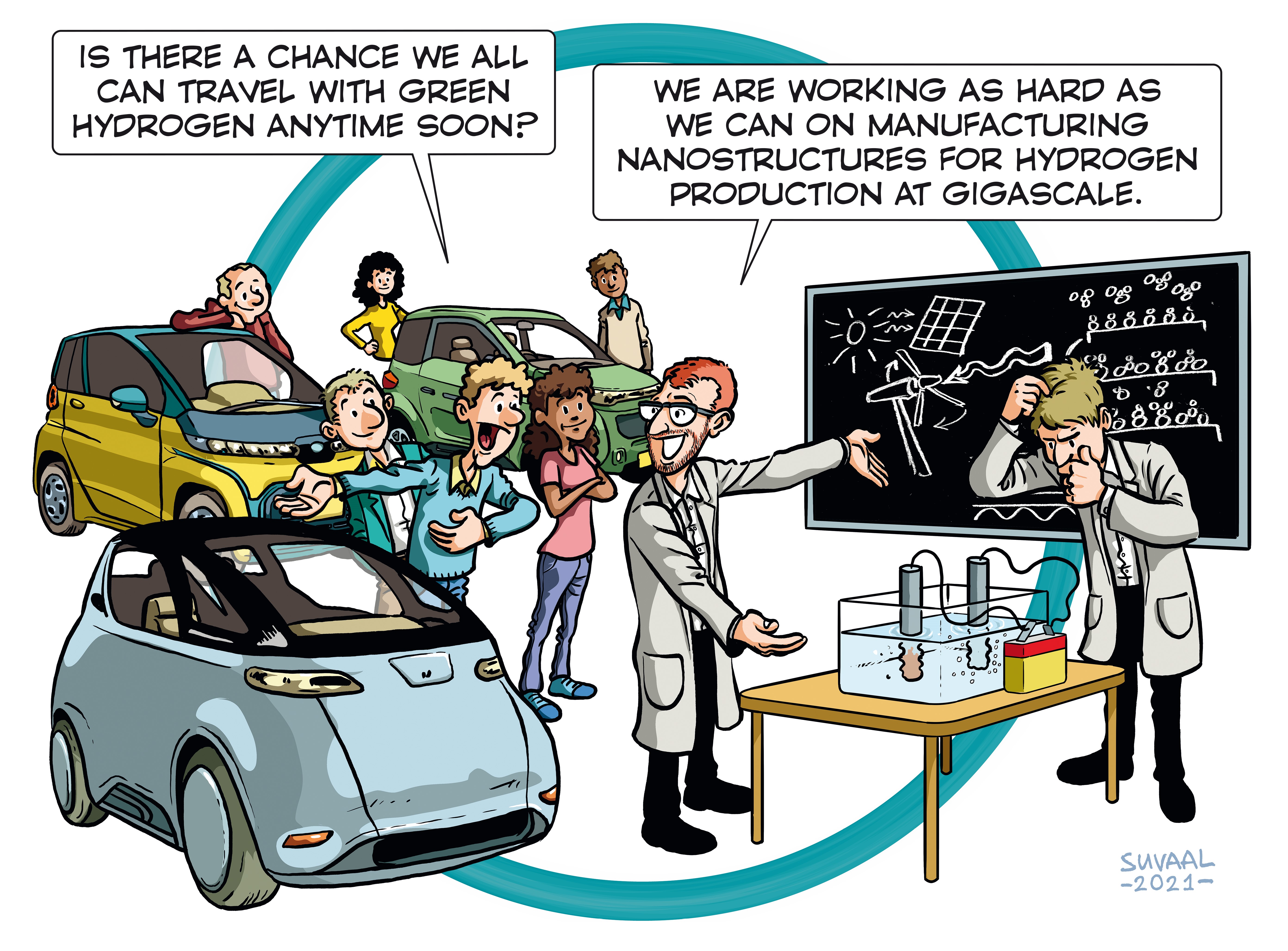
Hydrogen is created by putting electricity on a electrolysis cell filled with water. The water will be split into hydrogen and oxygen. Although this process is known for many years doing so on a large scale and in a sustainable way has many challenges.
The first challenge is how to perform electrolysis with renewable energy. The supply of renewable energy – solar or wind energy – depends very much on the weather – its availability fluctuates. For the longevity of the electrolyser you would want it to operate continuously and as efficiently as possible. Research is being done on how this can be achieved.
In order to perform electrolysis you need critical materials for the electrodes. This high demand forms the second challenge, as the availability of these materials - such as iridium - are scarce. Here the researcher is looking at how can we distribute this material as thinly as possible with nano layering technology so we can use it more efficiently and need less of the scares material per cell. Parallel to this he is also looking into the potential of using other materials for the electrodes
The third challenge is to find smart ways to scale up the size of the electrolysers in three dimensions. An electrolyser works with a fixed distance between the two electrodes. So, increasing the distance between the rods won’t suffice – excluding one dimension. You could increase the size of the electrodes however you are producing H2 gas which will rise to the top of the electrolyser, and from some height on the gap between the electrodes will become completely gas-filled, leaving no space for water. So there is a maximum height excluding another dimension. You could repeat the two rods after each other expanding in the width of the electrolyser but also here you need to think about how this can be done efficiently – also taking into consideration that the bubbles you are producing can stick to the electrodes interfering with the process. Research is being done if using a corrugated “cardboard-like” structure for the electrodes might be a solution to control the bubble flow.
What's next?
For hydrogen the next step is to build installations on industrial scale. Another next step is to see if a renewable fuel can by produced directly from CO2 using electrolyse technology or by combining hydrogen directly with CO2 using catalysts.
Contribution to the Energy transition?
We need a conversion in order to store renewable energy. Batteries are very costly to store huge amounts of energy. So we are looking for methods to chemically store large amounts of energy of which Hydrogen is one of the possibilities.
Prof. dr. ir. Ruud van Ommen
Faculties involved
- AS
