Moving ions around to turn CO2 into fuel
Imagine all CO2 emitted by the exhausts of cars being captured and reused to produce synthetic fuel, eventually resulting in zero net CO2 emission. This is what PhD student Eszter Mádai in the Materials Science & Engineering department of TU Delft is working on. DelftBlue has been indespensible in her work on two-dimensional electrodes that help reuse CO2. As she is nearing the end of her PhD project, she looks back at her experiences with the supercomputer.
Difficult to break
Mádai: “What makes it so difficult to reuse CO2 is that it is a very stable molecule. It’s difficult to break it up and turn it into other molecules. That’s why we need to add electrical energy and use a catalyst. All candidate catalysts need to be tested against criteria such as cost efficiency, environmental friendliness, and availability. For example, copper can break the bonds in the CO2 molecule very easily, but results in 16 different products, which need to be separated.”
Intercalating ions
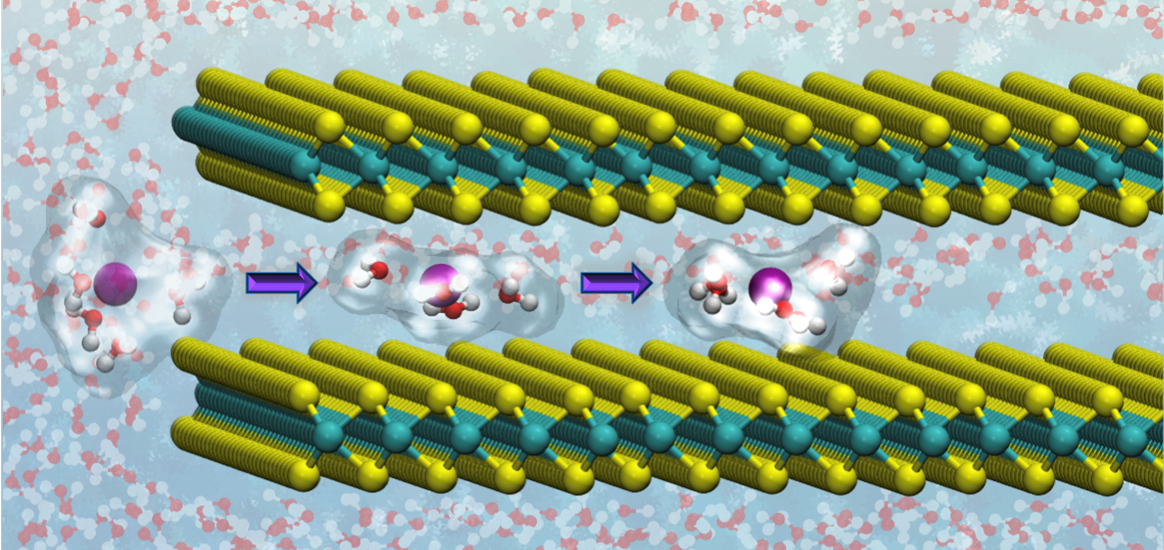
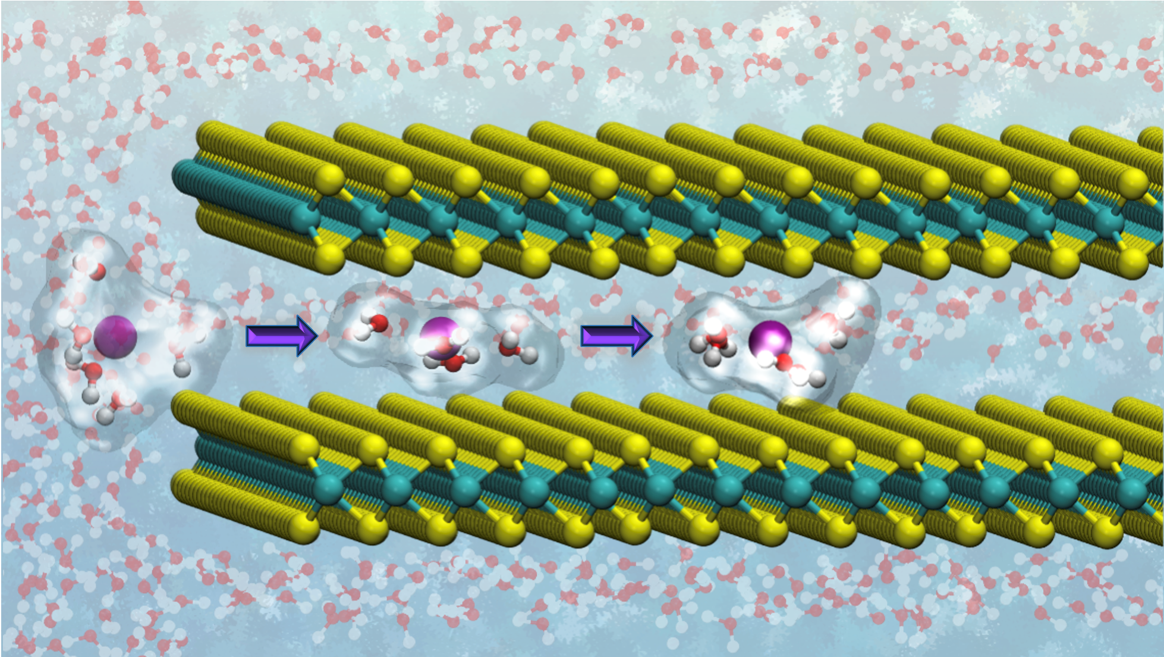
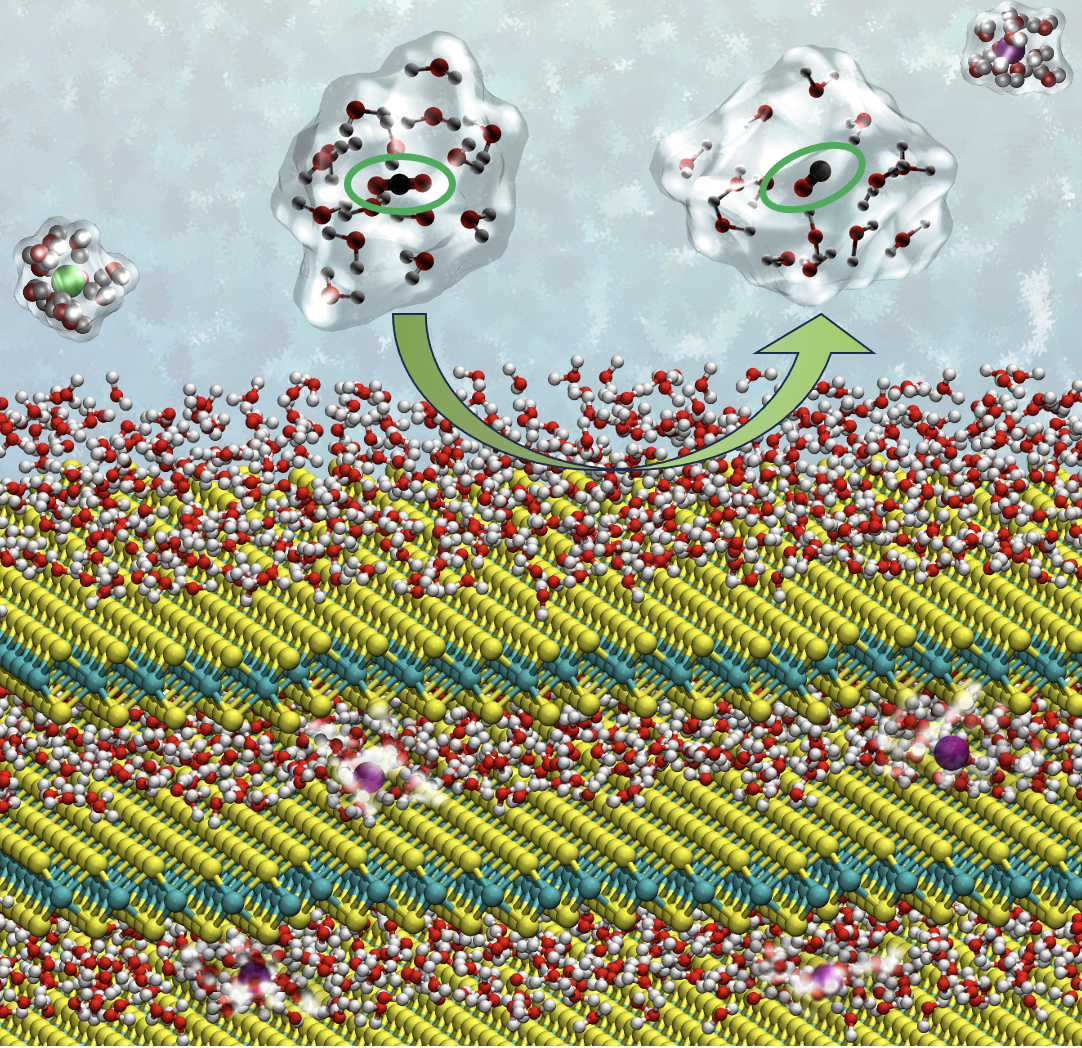
Mádai’s material of choice was molybdenum sulfide, consisting of stacked two-dimensional monolayers. “As the reaction with the CO2 takes place at the surface of the reactive material, we need a two-dimensional material structure with a high surface-to-bulk ratio.” But molybdenum sulfide will also split the water molecules accompanying the CO2 molecules, into hydrogen and oxygen. “In order to promote the CO2 reduction, I intercalated alkali metal ions in between monolayers of molybdenum sulfide.” That’s where DelftBlue came in.
Molecular dynamics simulations
Mádai used DelftBlue for Molecular Dynamics simulations, taking an ion and moving it in between the molybdenum sulfide layers, calculating for each position how much the ion likes to be there. The simulations revealed that applying an electric field is crucial in getting the ion into the right position. DelftBlue found the optimal potential for electrochemically intercalating the ion. “The Molecular Dynamics simulations supported our experimental work by providing an atomistic picture.”
Introductory course
“Using DelftBlue was an amazing experience, because it is so powerful”, explains Mádai. “As one of the first ‘regular’ users after the beta-tests, I felt the introductory course was particularly useful. The added value of the course is the personal interaction. If you are not familiar with Unix systems, it could be hard to get acquainted to the Linux language used by DelftBlue . The course teaches you exactly what the different commands are.”
Optimizing the speed
“The course also inspired me to try out new things. For example, I learned how to optimize the speed of the simulation or calculation with the number of nodes. Because at some point, it doesn't matter how many nodes you are using, the simulation won't get faster. That was really, really useful.”
Word of advice
Looking back, Mádai concludes that the simulations were a success. “Our original idea was to focus on macro-scale experiments, but DelftBlue allowed us to simulate what is happening in much more detail.” When asked to share a word of advice to people who are thinking about using DelftBlue, Mádai answers: “Take the course. Really, if you just start using the supercomputer, then you don't per se know how to use it efficiently.”