More efficient electrodes for CO2 recycling
With the ever-increasing interest in renewable energy, scientists are continuously searching for new technologies to store energy. CO2 electrolysis is a promising way to store energy whilst recycling carbon dioxide. By applying electricity, CO2 and water react and produce more complex molecules. A study published in Nature Communications lead by Hugo van Montfort at TU Delft has presented a new design of electrodes that improves the efficiency of CO2 electrolysis.
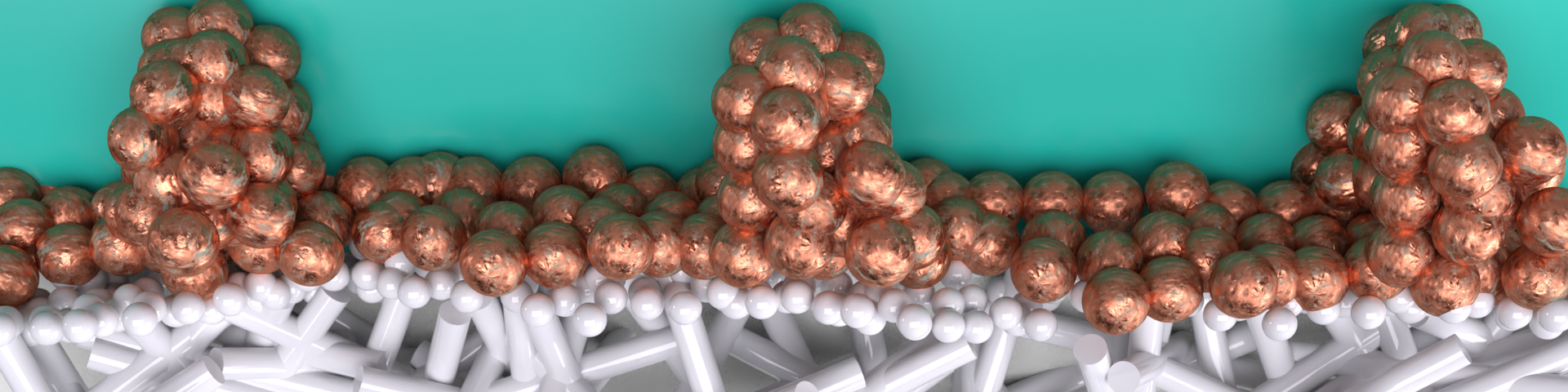
The currently much used expanded Teflon electrodes (ePTFE) are porous, meaning that they have holes inside their structure. This allows the gaseous CO2 to travel to where the reaction happens, leading to a rapid product formation in the flow cell; the device in which electrolysis takes place.
“On the electrode, the applied current is transformed into chemical bonds using a catalyst, in this case copper”, PhD candidate Van Montfort explains. “The ePTFE electrodes have been used to improve the stability of the flow cells without anyone really looking at the constraints that these electrodes put on the design. We looked at how different loadings of catalyst influence the distribution of the electrical current. We were able to show that an alternative way of distributing this current increases the overall yield of the reaction.”
Electrodes in new (infrared) light
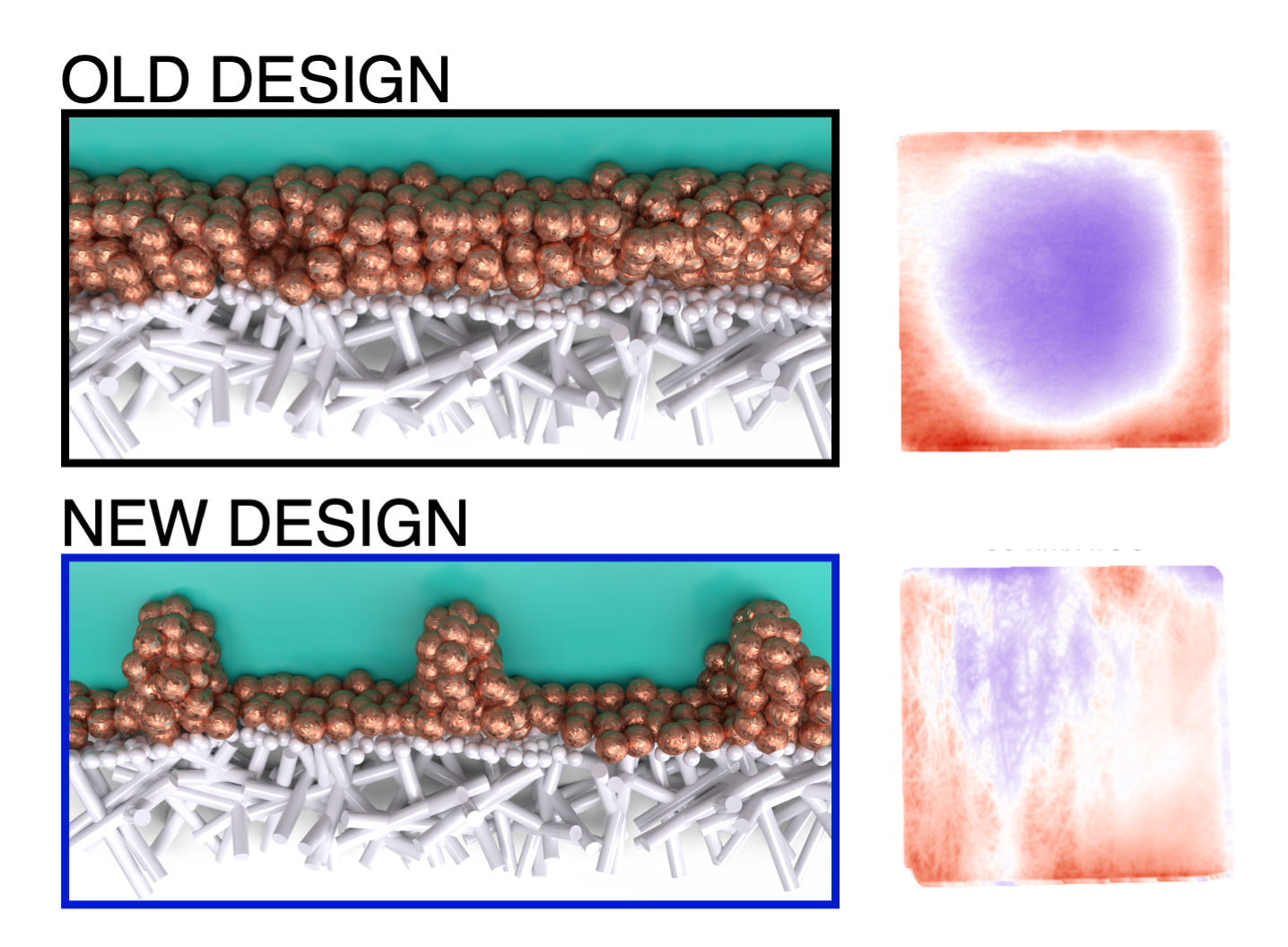
“We saw that if the catalyst layer on these electrodes is very thin, the current concentrates on the edges of the electrode. This makes scaling up these systems difficult, because larger electrodes will then require thicker and thicker catalyst layers to spread the current over the surface. By engineering a new design for current distribution, we showed how we can improve this distribution without affecting the product mix.”
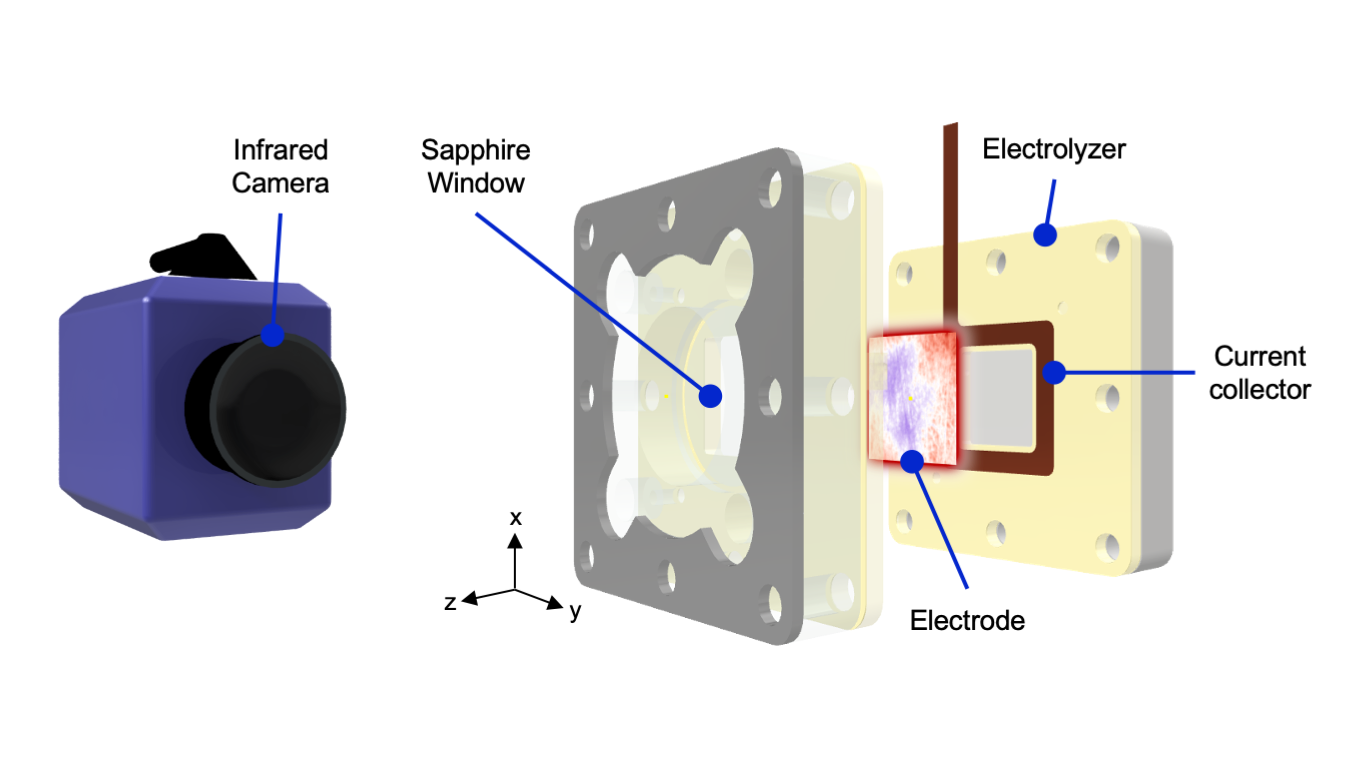
The research offers quite literally a new way of looking at these electrodes: “By using infrared cameras to study them, we can see how hot each part of the electrode becomes. The warmer parts experience a higher current, indicating that the reaction occurs more rapidly there”, Van Montfort adds.
We have good hope that this technology is only a few decades away from making a difference on the industrial scale.
Making a difference
CO2 electrolysis offers two enormous benefits as a technology for energy production. First of all, it is a carbon-neutral way of producing complex molecules, such as ethylene and methane. These substances are used in the chemical industry for the production of plastics, textile and other materials. These molecules would normally have to be extracted from gas, or oil. The scaling of this technology would thus reduce the use of fossil fuels. Moreover, this technology only relies on water and carbon dioxide, a molecule that is produced as waste in many industrial processes and of which there is an abundance in the atmosphere already.
“Although CO2 electrolysis is one of the technologies poised to supply carbon-neutral chemicals in the near future, there are still serious limitations that prevent its widespread application in the chemical industry”, Van Montfort says. “Our new design does not solve all the problems that are associated with this technology. Nonetheless, we have good hope that it is only a few decades away from making a difference on the industrial scale.“
(Scientific) word spreads fast
“The research published has been a greatly international collaboration”, Van Montfort adds. “We collaborated with a group in Australia that is experienced in imaging very tiny objects using scanning-electron microscopes. This work is a team effort, and every single co-author has contributed to the article. And, on the other hand, news seem to travel fast in the science world. We’ve been invited to give talks about our findings and people are asking us to explain our technique and collaborating with them.”

Hugo-Pieter Iglesias van Montfort
PhD Candidate