Delft scientists put the spotlight on combined capture and conversion of CO2
CO2 can be electrochemically converted into valuable chemicals and fuels. Both capturing and converting CO2 do, however, require a lot of energy. An optimal combination of both processes can save a lot of energy and reduce the loss of materials. The TU Delft research group of David Vermaas recently published a paper in Nature Catalysis, summarising the various ideas on how to achieve this. What are the opportunities, the bottlenecks? And what options are feasible?
Glasgow
CO2 emissions play an important role in the climate crisis. Reducing CO2 emissions therefore was a recurring topic during the UN Climate Change Conference. But capturing and converting CO2 is an equally important alternative because, for the foreseeable future, we will continue to depend on processes requiring hydrocarbons. Think of materials such as cement, plastics, and chemicals, but also of long-distance mobility. There is a lot of room for improvement, specifically when it comes to CO2 capture and conversion, Vermaas says.
Three options
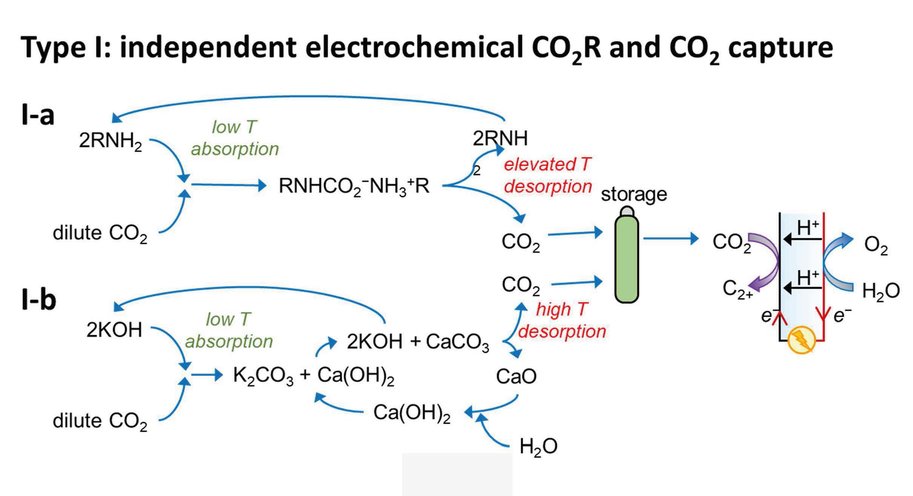
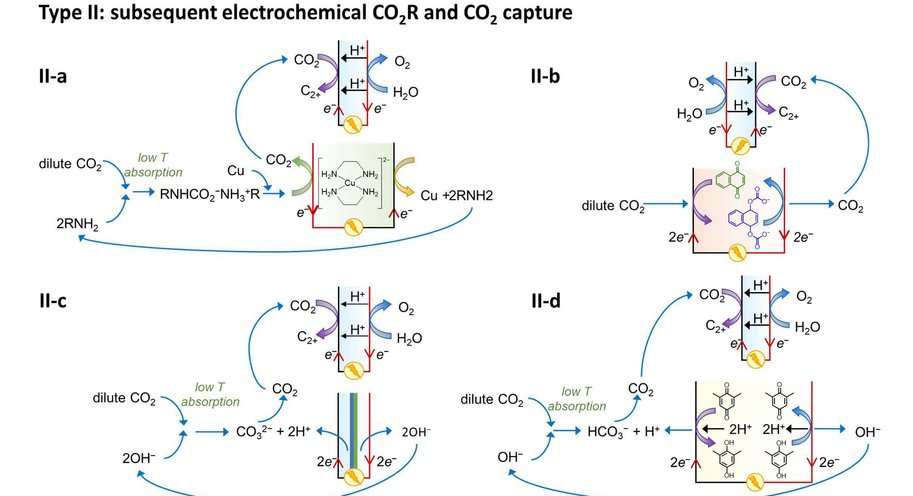
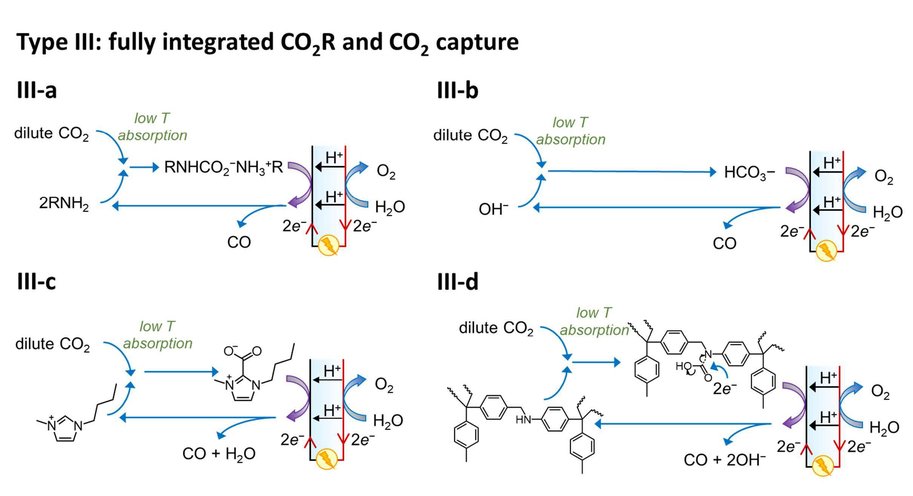
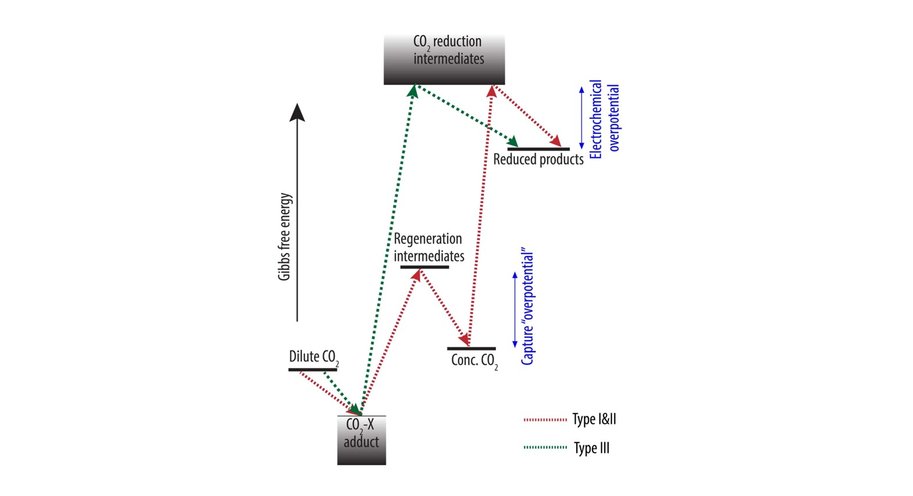
At the moment, capturing CO2 and its electrochemical conversion are both very energy intensive processes. A synergistic merging of the two processes can increase overall energy efficiency and reduce costs. Vermaas explains it like this: ‘In our publication we consider three options for such merging. First, we can put both techniques in sequence. The first machine captures CO2, and the second machine converts it. This is the easiest and most obvious method, but it comes with no gains: you still need to extract the CO2 from the liquid used for capturing it. The second option comes down to combining the two separate reactions (CO2 capture and its conversion) in a single machine, allowing some material savings. The third option is to make the CO2 part of the chemical reaction while it is still solved in the liquid (as a so-called CO2 adduct).’
Energy savings
The paper – by Delft scientists in collaboration with researchers from the California Institute of Technology (Caltech) – estimates the potential energy savings of merging CO2 capture with CO2 conversion. ‘In theory, it costs about 20 kJ of energy to extract a mole of CO2 from air or water. In practice, when doing this in a separate step, it takes 100-300 kJ/mole, because you are dealing with both the CO2 and all the liquid containing it. By having the CO2-complexes take direct part in the chemical reaction, you can reach this thermodynamic limit of 20 kJ/mole, on top of the usual energy needed to convert the CO2. This is a big step forward because energy use is the main bottleneck for capturing CO2.’
Puzzle
There is still a long way to go. ‘We have very little knowledge of the reaction mechanisms involved in this third route, in which the CO2-complexes take direct part in the chemical reaction,’ Vermaas explains. ‘We also don’t yet know which catalysts to use. In theory, because of the much higher CO2 concentration, this route should enable extremely high current densities – and therefore a high conversion rate (amount of CO2 converted each second). It is unclear to me why we are unable to achieve this in practice, but we do need to increase this reaction speed. I am fascinated by this scientific puzzle.’
Diffuse
CO2 can be captured from both point sources and diffuse sources. The chimney of a power plant is an example of a point source, in which CO2 is released from a single, identifiable location. It is relatively easy to deal with such point sources. A diffuse source, on the other hand, is much harder to deal with. Think of CO2 emitted by crops and cattle, or of exhaust fumes from transportation and residential heating systems.
Vermaas: ‘At the moment, diffuse sources account for forty percent of CO2 emissions. This share is expected to increase as more and more coal power plants and high-polluting factories are being replaced by renewables, while diffuse emitters are more difficult to replace. One way or another, there will always be a need to capture CO2 from diffuse sources – from the air or sea water. Subsequently, this CO2 will need to be converted to something more useful. The world aims to be CO2-neutral by 2050, but ten to fifteen percent of our global energy demand will continue to be met with traditional fuels. This means that we will have to capture and convert its CO2 equivalent. We haven’t yet reached the point where this is possible. That’s why this research is so incredibly important.’

Dr.ir. David Vermaas
Contact
For press questions, please contact
- Dave Boomkens, Energy Transition science information officer at TU Delft – D.J.Boomkens@tudelft.nl and +31 (0) 6 34081461
- David Vermaas, assistant professor Electrochemical Systems at TU Delft – D.A.Vermaas@tudelft.nl and +31 (0) 6 14310197
Follow this link to read the publication by the research group of David Vermaas and his colleagues from Caltech.