Experimental insights into anodic oxidation of hexafluoropropylene oxide dimer acid (GenX) on boron-doped diamond anodes
GenX is an emerging pollutant which belongs to a family of substances called PFAS. They are commonly nicknamed ‘forever chemicals’ owing to their high chemical stability, inability to degrade naturally, and resistance to conventional treatment methods. PFAS are omnipresent and there is evidence that PFAS exposure can lead to adverse health outcomes in humans, including liver damage and cancer. Till date, very limited research is reported on HFPO-DA (GenX) degradation.
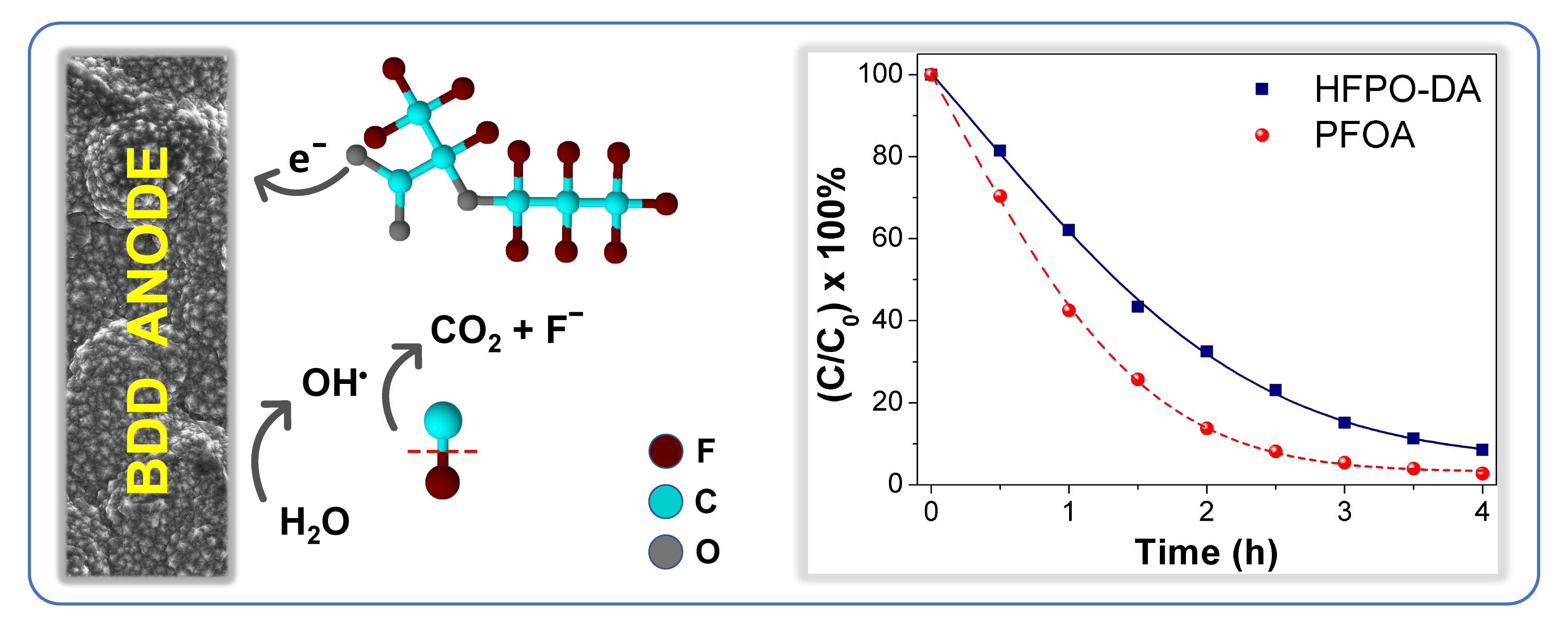
A team of TU Delft researchers (Ivan Buijnsters, Arjan Mol and Diwakar Suresh Babu – now PhD student at Helmholtz-Zentrum Berlin) carried out an experimental study to investigate the degradation and defluorination efficiency of GenX using boron-doped diamond anodes in electrochemical advanced oxidation processes. Their study aimed to elucidate the first step in the degradation mechanism of HFPO-DA and to investigate the influence of sulfate concentration and other operating parameters on HFPO-DA degradation.
Results demonstrated that sulfate radicals were ineffective in HFPO-DA degradation due to steric hindrance by –CF3 branch. Direct electron transfer was found as the rate-determining step. A degradation pathway was proposed in which HFPO-DA mineralizes to CO2 and Fˉ via formation of three intermediates. Using boron-doped diamond electrodes which generate highly reactive hydroxyl radicals, a 100% degradation of GenX in aqueous solution was achieved.
Main findings of their study were recently published in the journal Chemosphere (https://doi.org/10.1016/j.chemosphere.2021.132417).

