Innovative nanotransistor for easy measurement of electrolyte concentration in blood
Testing the blood of patients that doctors believe may be suffering from an electrolyte imbalance is usually a major effort because various selective tests have to be performed. Electrolytes are certain nutrients or chemicals in the body that carry out a number of important functions, such as regulating the heartbeat. A disruption of the electrolyte balance can be dangerous. Researchers working in the field of chemistry are examining the chemical compounds of electrolytes, which are partly split into ions and conduct electrical currents. Remco Hartkamp, assistant professor of computational chemical physics at the Department of Process & Energy, developed a new method, together with researchers from the Centre National de la Recherche Scientifique (CNRS) in France and the NTT Basic Research Laboratories in Japan, that will make it easier to measure the concentration of different electrolytes in the body using a nanotransistor. The results of the research were published this month in the Nature Materials: ‘Selective layer-free blood serum ionogram based on ion-specific interactions with a nanotransistor’.
Summary ‘Selective layer-free blood serum ionogram based on ion-specific interactions with a nanotransistor’
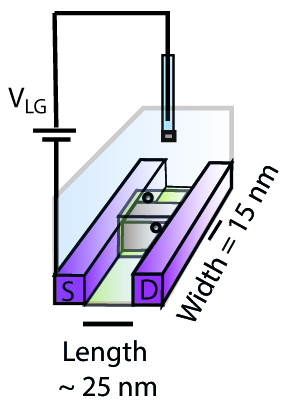
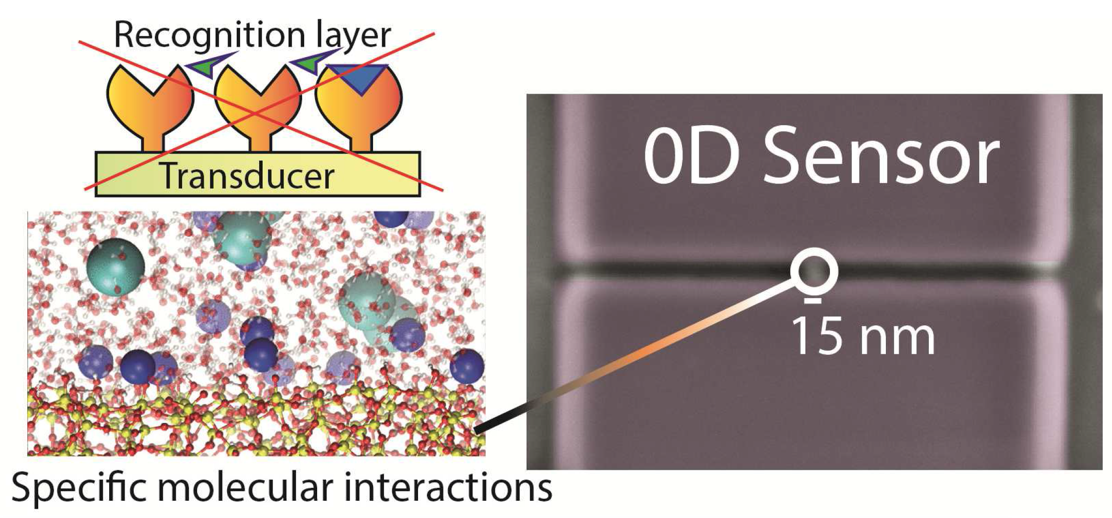
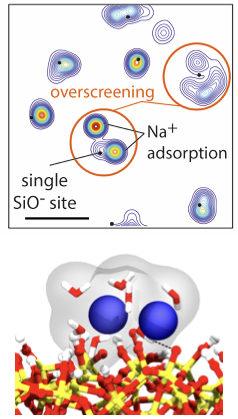
The researchers presented what they called a ‘0D’ ion-selective field-effect transistor (ISFET), with a sensor of just 25 nm - much smaller than the conventional nanowire ISFETs. Compared to currently available devices, the 0D-ISFET showed a qualitatively different response to changes in pH and a much higher sensitivity to the presence of electrolytes, making it possible to sense electrolytes that are present in very small concentrations. Combining the experiments with molecular dynamics simulations and site-binding theory, new insight was acquired. Specifically, a theoretical approach to the trends found in the experimental measurements suggested an additive, rather than competitive, effect of mixed electrolytes on the electric potential at the solid-liquid interface. Furthermore, simulations suggested that the high sensitivity of the 0D-ISFET, especially with respect to divalent ions, may be caused by an excess of cations adsorbing onto the charged solid surface. Such overscreening is ascribed to a combination of electrostatic correlations and ion-specific adsorption.
The 0D-ISFET can facilitate much cheaper and easier bedside testing, with only a tiny amount of blood needed. These developments are particularly important for patients who undergo regular ionogram measurements (for hyperkalemy or renal insufficiency) or who take antidiabetic, corticoid or lithium medications. Apart from the envisioned application in blood analysis, gaining an improved molecular-level understanding of ion specificity at the solid-electrolyte interface is important to a plethora of other applications. The marriage between experiments, simulations and theory is pivotal in the quest for gaining this molecular-level insight. The present collaborative study has taken important steps towards more accurate and versatile blood analysis at low cost and has improved fundamental understanding of ion-specific adsorption. Regardless, much more is to be learned both on the fundamental and applied side and further study is being performed to complement the present data and build on the current findings.
Read more about Remco Hartkamp