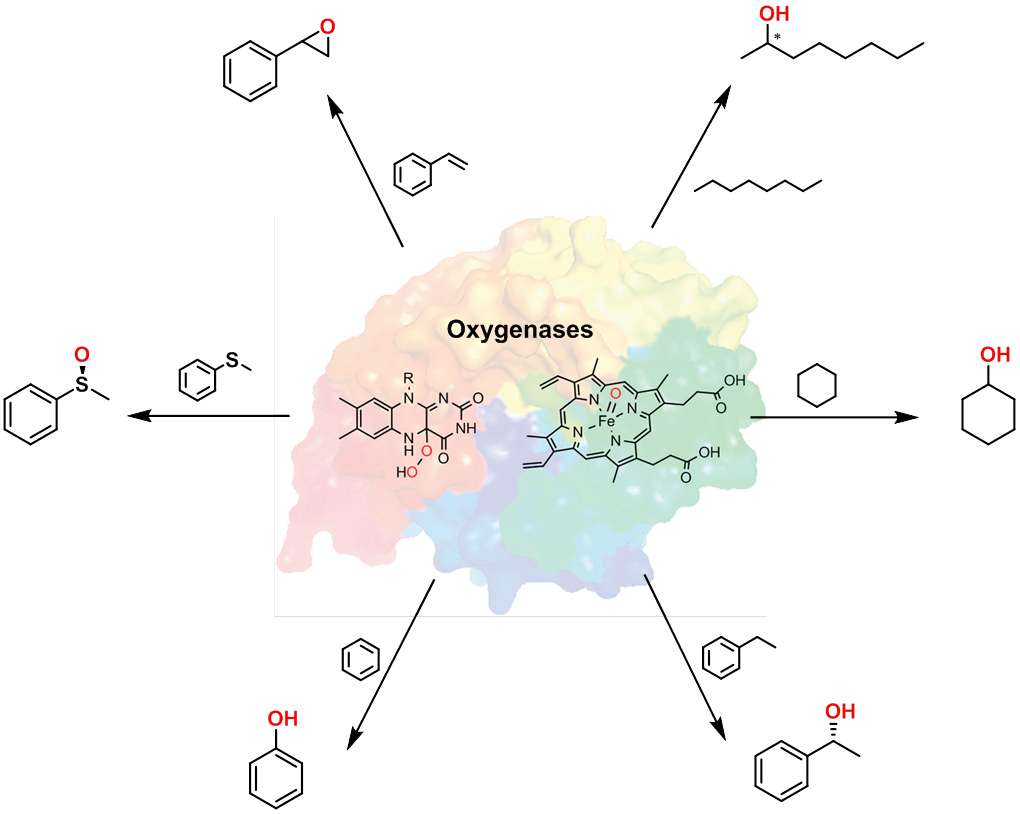
Nature uses enzymes to catalyze a wealth of biotransformations. However in practice these transformations are carried out in-vivo under conditions which are far from suitable for large scale production of chemicals. It is our challenge to engineer or design enzymes in such a way that they can be employed in-vitro as catalysts.1,2 E.g. in the case of oxidations, the power of enzymes can circumvent the use of halogen-containing and environmentally demanding oxidants, by using oxygen or hydrogen peroxide as clean and selective oxidants. Different classes of enzymes can be employed as highly promising catalysts for biocatalytic oxidation of alkenes, alkanes and alcohols.
A notorious example from our lab from 2010 is the laccase/TEMPO system which is a practical approach towards selective oxidation of alcohols (insert pubs). Both the mechanistic aspects3 as well an optimized procedure using immobilized laccase4 were published following the original discovery.
- F. Hollmann, I.W.C.E. Arends, K. Buehler, A. Schallmey and B. Bühler, Enzyme-mediated oxidations for the chemist, Green Chem. 13 (2011) 226-265.
- D. Holtmann, M.W. Fraaije, I.W.C.E. Arends, D.J. Opperman and F. Hollmann, The taming of oxygen: biocatalytic oxyfunctionalisations, Chem. Commun. 50 (2014) 13180-13200.
- S.A. Tromp, I. Matijosyte, R.A. Sheldon, I.W.C.E. Arends, G. Mul, M.T. Kreutzer, J.A. Moulijn and S. de Vries: Mechanism of Laccase/TEMPO-catalyzed oxidation of benzyl alcohol, ChemCatChem 2 (2010) 827-833.
- I. Matijosyte, I.W.C.E. Arends, S. de Vries and R.A. Sheldon: Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases, J. Mol. Catal. B: Enzymatic 62 (2010) 142-148.