Research
Organic Functional Molecular Materials and Devices for Advanced Energy Applications
The main theme of our research is the design, synthesis and characterization of functional organic molecules and materials for advanced energy applications. The molecules we are currently working on mostly, are perylene-3,4,9,10-tetracarboxylic acid derivatives, PTCAs in short.
These organic dye molecules exhibit outstanding physical properties, such as chemical robustness, thermal, photochemical and electrochemical stability, along with excellent optical and electronic properties. These compounds have been applied as industrial colorants, fluorescent sensors, and n-type semiconductors in the field of organic electronics and photovoltaics. From a chemical perspective, PTCAs are extremely versatile compounds that offer plenty opportunities for chemical modifications. These modifications enable a systematic tuning of their optoelectronic properties.
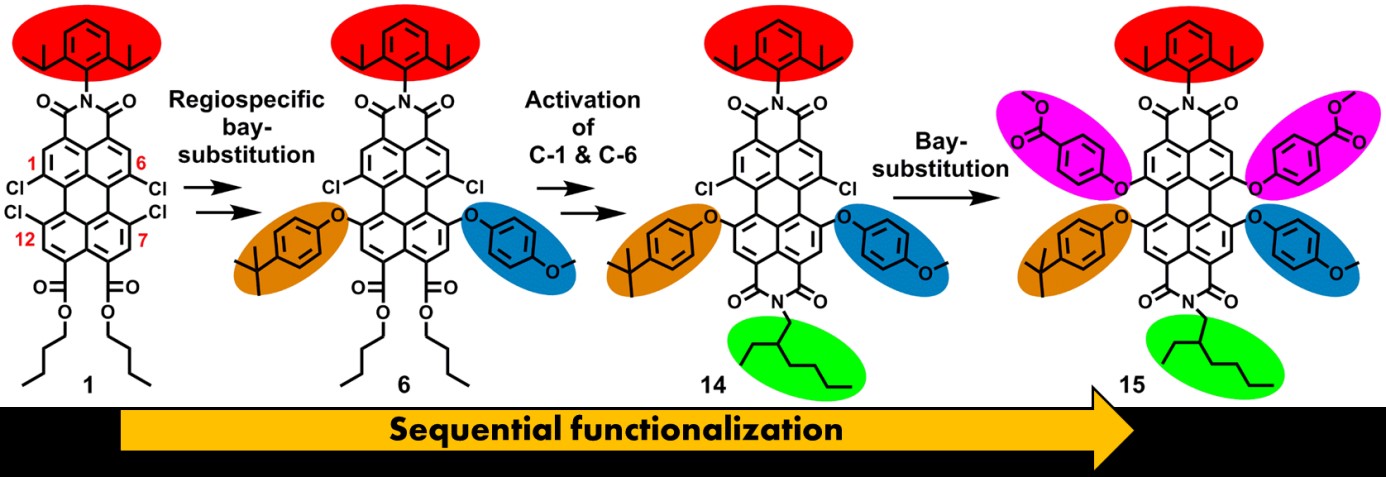
In recent years we have developed methodologies for synthesizing PTCAs, using flexible and scalable synthetic methods, aiming at the attachment of functional groups at well-defined positions. Currently, we are able to attach five different substituents at independent positions at the perylene core.
Application of these molecules or of molecular devices derived thereof is focused on artificial photosynthesis, electroactive polymers for batteries and fluorescent probes. The major research activities are designing novel molecules, developing pathways for efficient synthesis, investigating structure property relationship of molecules and materials and incorporation in molecular devices.
Our research is divided into four distinct somewhat overlapping topics
Exploration and development of novel synthetic methods
Development of photoactive molecules and materials, notably for applications in artificial photosynthesis (with Ferdinand Grozema, ChemE/OM)
Development of molecular fluorescent probes (with biomedical applications in mind)
Development of molecules and materials for electrochemical applications, notably alkali-ion batteries (with Erik Kelder and Marnix Wagemaker, RST/SEE)
Selected publications
Subject 1:
Highly Efficient Synthesis of Regioisomerically Pure 1,7-Dibromo Perylene-3,4,9,10-Tetracarboxylic Acid Derivatives. Sengupta, S; Dubey, R. K.; Hoek, R. W. M.; van Eeden, S. P. P.; Gunbaş, D. D; Grozema, F. C.; Sudhölter, E. J. R.; Wolter F. Jager. J. Org. Chem. 2014, 79, 6655-6662.
Novel Derivatives of 1,6,7,12-Tetrachloroperylene-3,4,9,10-Tetracarboxylic Acid: Synthesis, Electrochemical and Optical Properties. Rajeev K. Dubey, Nick Westerveld, Ernst J. R. Sudhölter, Ferdinand C. Grozema and Wolter F. Jager. Org. Chem. Frontiers, 2016, 3, 1481-1492.
Synthesis of Perylene-3,4,9,10-tetracarboxylic Acid Derivatives Bearing Four Different Substitutions at the Perylene Core. Dubey, R. K.; Westerveld, N.; Eustace, S. J.; Grozema, F. C.; Sudhölter, E. J. R.; Wolter F. Jager. Org. Lett. 2016, 18, 5648-5651.
Perylene Bisimide Dyes with Up to Five Independently Introduced Substituents: Controlling the Functionalization Pattern and Photo-physical Properties Using Regiospecific Bay-Substitution. Dubey, R. K.; Eustace, S. J.; van Mullem, J. S.; Sudhölter, E. J. R.; Grozema, F. C.; Jager, W. F. J. Org. Chem. 2019, 84.
Subject 2:
Tunable and Highly Efficient Light-Harvesting Antenna Systems Based on 1,7-Perylene-3,4,9,10-Tetracarboxylic Acid Derivatives. Dubey, R. K.; Inan, D.; Sengupta,S.; Sudhölter, E. J. R.; Grozema, F. C.; Jager, W. F. Chem Sci. 2016, 7, 3517-3532.
Substitution Effects on the Photoinduced Charge-Transfer Properties of Nover Perylene-3,4,9,10-tetracarboxylic Acid Derivatives. Inan, D.; Dubey, R. D.; Westerveld, N.; Bleeker, J.; Jager, W. F.; Grozema, F. C. J. Phys Chem. A, 2017, 121, 4633-4644.
Tailoring Photophysical Processes of Perylene-Based Light Harvesting Antenna Systems with Molecular Structure and Solvent Polarity. Inan, D.; Dubey, R. K.; Jager, W. F.; Grozema, F. C. J. Phys. Chem. C. 2019, 123, 36-47.
Subject 3:
7-Dialkylamino-1-alkylquinolinium Salts; Highly Versatile and Stable Fluorescent Probes. van den Berg, O.; Jager, W.F.; Picken, S.J. J. Org. Chem. 2006, 71, 2666-2676.
Highly Sensitive Water-soluble Fluorescent pH Sensors Based on the 7-Amino-1-methyl-quinolinium Chromophore. Jager, W.F.; Hammink, T.S.; van den Berg, Grozema, F.C. J. Org. Chem.2010, 75, 2169-2178.
Fluorescent PET probes based on perylene-3,4,9,10-tetracarboxylic tetraesters. Dubey, R. K.; Knorr, G.; Westerveld, N.; Jager, W. F. Org. Biomol. Chem. 2016, 14, 1564-1568.
Exploration and development of novel synthetic methods
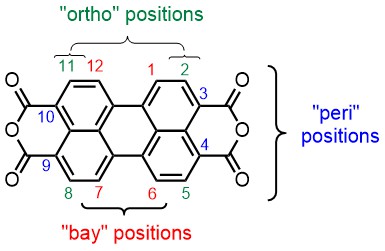
The first topic is pretty much a stand-alone topic in which we strive at finding methods to add independent substituents to the bay- and peri positions of the perylene core. In the scheme above it is illustrated how five independent substituents are attached. Naturally, getting the sixth substituents on is an ambition for the near future, but we are also very much interested in bringing the ortho-substituents in the game.
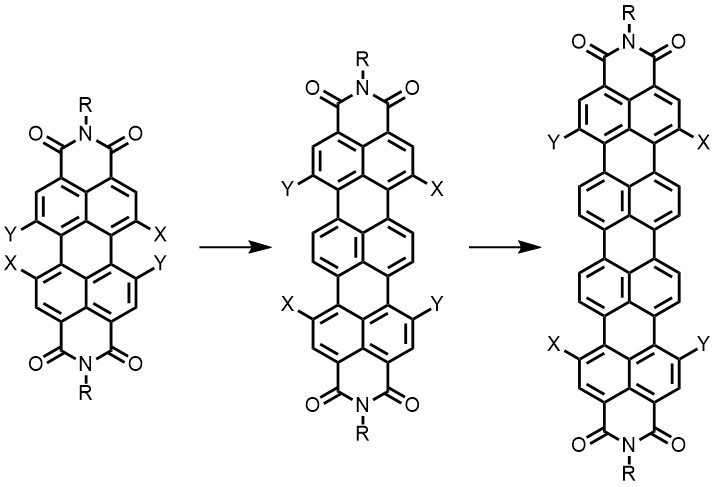
Expanding the p-system of perylenes and developing higher rylenes, without compromising the solubility too much, and attaching substituents to these rylenes is another goal within topic 1. The higher rylenes absorb and emit at higher wavelength and are thus interesting for artificial photosynthesis and fluorescent probing.
References:
Perylene Bisimide Dyes with Up to Five Independently Introduced Substituents: Controlling the Functionalization Pattern and Photo-physical Properties Using Regiospecific Bay-Substitution. Dubey, R. K.; Eustace, S. J.; van Mullem, J. S.; Sudhölter, E. J. R.; Grozema, F. C.; Jager, W. F. J. Org. Chem. 2019, 84.
Pentarylene- and Hexarylenebis(dicarboximide)s: Near-Infrared-Absorbing Polyaromatic Dyes. Pschirer, N. G.; Kohl, C.; Nolde, F.; Qu, J.; Müllen, K. Angew. Chem. Int. Ed. 2006, 45, 1401-1404.
Highly Efficient Synthesis of Regioisomerically Pure 1,7-Dibromo Perylene-3,4,9,10-Tetracarboxylic Acid Derivatives. Sengupta, S; Dubey, R. K.; Hoek, R. W. M.; van Eeden, S. P. P.; Gunbaş, D. D; Grozema, F. C.; Sudhölter, E. J. R.; Wolter F. Jager. J. Org. Chem. 2014, 79, 6655-6662.
Novel Derivatives of 1,6,7,12-Tetrachloroperylene-3,4,9,10-Tetracarboxylic Acid: Synthesis, Electrochemical and Optical Properties. Rajeev K. Dubey, Nick Westerveld, Ernst J. R. Sudhölter, Ferdinand C. Grozema and Wolter F. Jager. Org. Chem. Frontiers, 2016, 3, 1481-1492.
Synthesis of Perylene-3,4,9,10-tetracarboxylic Acid Derivatives Bearing Four Different Substitutions at the Perylene Core. Dubey, R. K.; Westerveld, N.; Eustace, S. J.; Grozema, F. C.; Sudhölter, E. J. R.; Wolter F. Jager. Org. Lett. 2016, 18, 5648-5651.
Development of photoactive molecules and materials, notably for applications in artificial photosynthesis
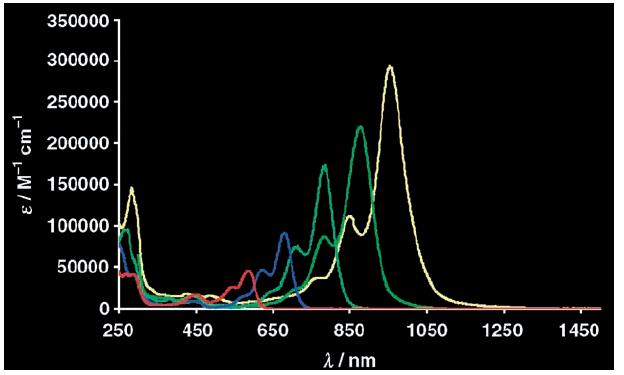
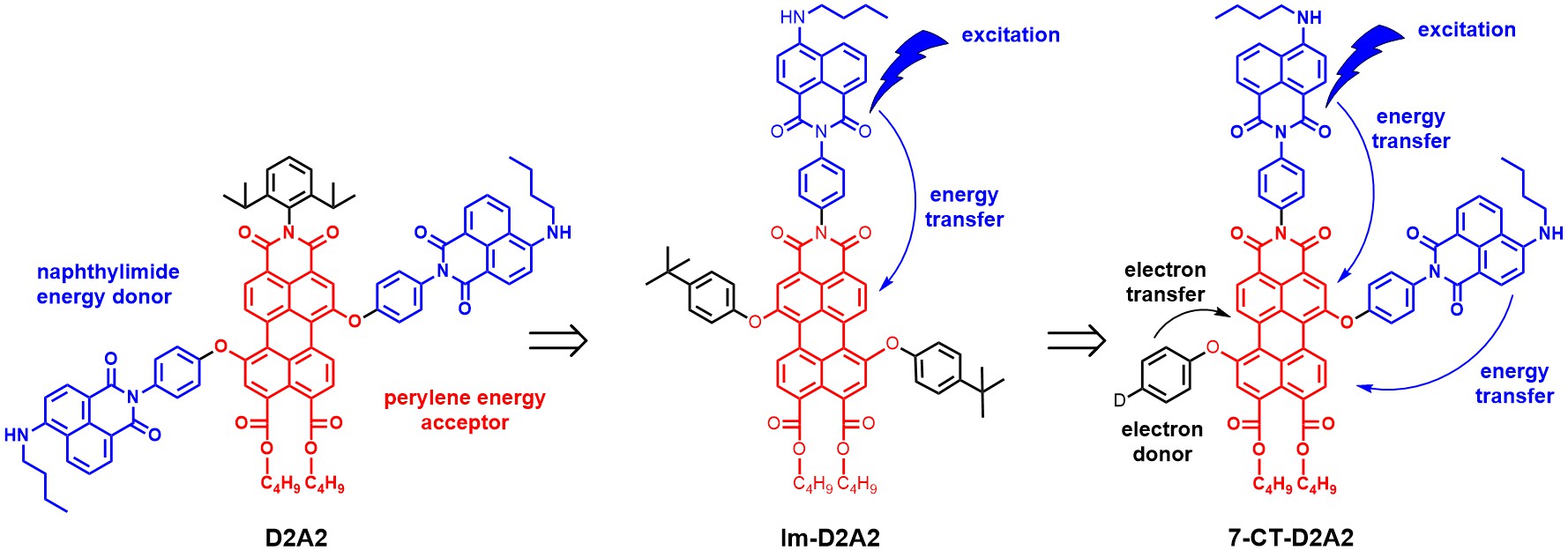
In topic 2, synthetic methods are applied for the development of photoactive molecules and materials. Previous research has been focussed on attaching energy donors to the perylene core, as in D2A2 and Im-D2A2, whereas current research is shifting from excited energy transfer processes (EET) and focussing on photoinduced charge transfer. This is focussed on regiospecific attachment of electron donating groups in molecules, like in 7-CT-D2A2, which are polarized along the perylene long axis. Such molecules are capable of light harvesting and charge separation, as illustrated in the Figure below.
Finding the optimum location for electron donor attachment, developing donors for obtaining long-lived charge separated states and finding more polarized perylene scaffolds, in which a more “guided charge transfer is expected, are important research objectives at this moment.
Developing covalent organic frameworks (COFs) or metal organic frameworks (MOFs) in which PTCA linkages are incorporated is another emerging research topic in our labs. In these frameworks we expect that solution properties of the PTCAs are retained in the condensed phase and that excited energy transfer, i.e. light-harvesting, is a highly efficient process. With the synthesis of such frameworks, we move from photo-functional molecules to photofunctional materials for which applications in artificial photosynthesis are envisaged.
References:
Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Frischmann, P. D.; Mahata, K.; Würthner, F. Chem. Soc. Rev. 2013, 42, 1847−1870.
Tunable and Highly Efficient Light-Harvesting Antenna Systems Based on 1,7-Perylene-3,4,9,10-Tetracarboxylic Acid Derivatives. Dubey, R. K.; Inan, D.; Sengupta,S.; Sudhölter, E. J. R.; Grozema, F. C.; Jager, W. F. Chem Sci. 2016, 7, 3517-3532.
Substitution Effects on the Photoinduced Charge-Transfer Properties of Nover Perylene-3,4,9,10-tetracarboxylic Acid Derivatives. Inan, D.; Dubey, R. D.; Westerveld, N.; Bleeker, J.; Jager, W. F.; Grozema, F. C. J. Phys Chem. A, 2017, 121, 4633-4644.
Tailoring Photophysical Processes of Perylene-Based Light Harvesting Antenna Systems with Molecular Structure and Solvent Polarity. Inan, D.; Dubey, R. K.; Jager, W. F.; Grozema, F. C. J. Phys. Chem. C. 2019, 123, 36-47.
Development of molecular fluorescent probes
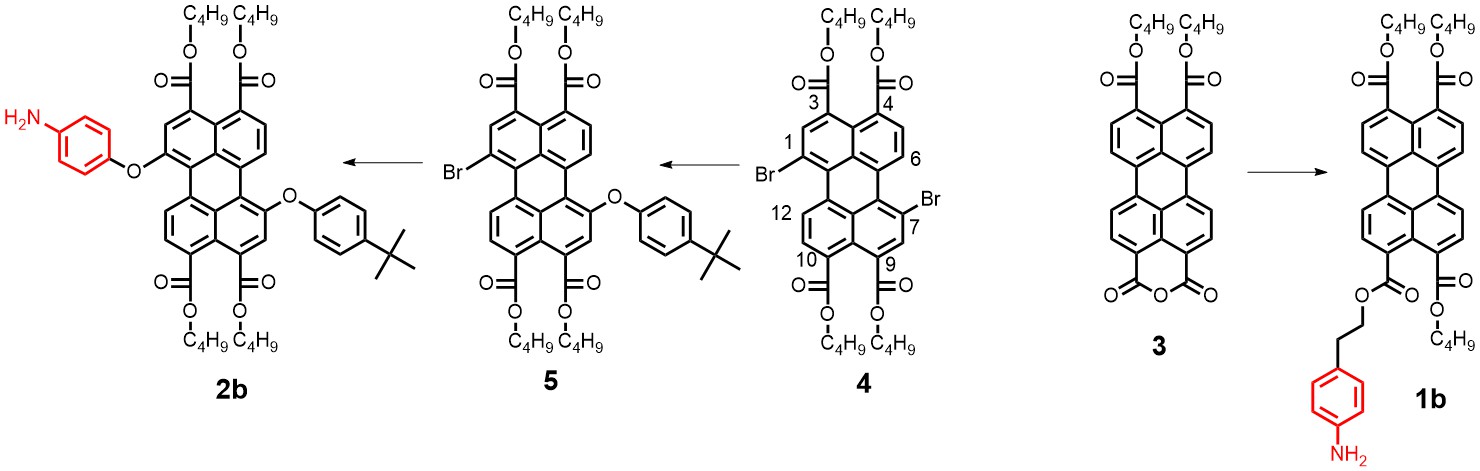
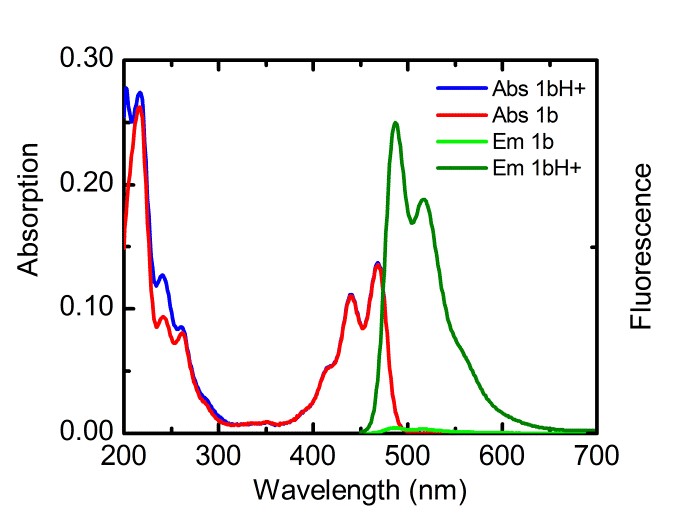
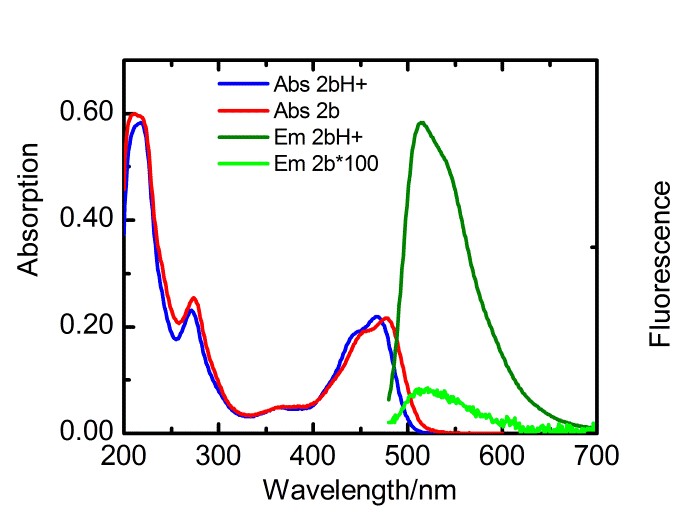
With respect to topic 3, we have developed the synthetic methodology to achieve independent substitution at the peri- and bay-positions of perylene tetraesters (PTEs), and have made efficient pH and ROX probes.1 PTEs are chosen as scaffolds for fluorescent probes because they are far more soluble in polar solvents than prylene bisimides (PBIs). These fluorescent probes, which modulate their fluorescence intensity by the photoinduced electron transfer (PET) principle,2 have been developed using aniline and piperazine as proton receptors and thiols for ROX detection. The syntheses of some probes is illustrated below.
Spectroscopic measurements show that the performance of bay-substituted PTEs like 2b is superior compared to peri substituted probes like 1b. Extremely high contrast upon protonation (FE >800) and near-quantitative fluorescence quantum yields (FF= 0.87) in the protonated state have been observed.
Current work is aimed at finding appropriate receptors to make probes sensitive in the proper concentration range, making probes water-soluble and probing different analytes simultaneously. These probes may be useful as molecular tools for spatiotemporal (bio)imaging.
References:
1: Fluorescent PET probes based on perylene-3,4,9,10-tetracarboxylic tetraesters. Dubey, R. K.; Knorr, G.; Westerveld, N.; Jager, W. F. Org. Biomol. Chem. 2016, 14, 1564-1568.
2: Daly, B.; Ling, J.; de Silva, A. P. Chem. Soc. Rev., 2015, 44, 4203-4211. Jager, W.F.; Hammink, T.S.; van den Berg, Grozema, F.C. J. Org. Chem.2010, 75, 2169-2178.
Development of molecules and materials for electrochemical applications, notably alkali-ion batteries
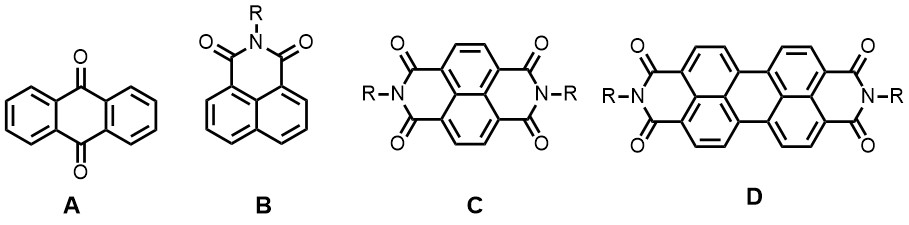
In topic 4, perylene and naphthalene (bis)imides (B-D), anthraquinones (A) and other redox-active organic molecules are employed as active electrode materials in batteries. Basically, in battery electrodes these molecules are reversibly reduced by taking up electrons and alkali metal ions, commonly at the carbonyl positions. Organic molecules for these applications are in high demand because they are tuneable, affordable and originate from a sustainable source. By modification of the molecular structure the redox potential (the battery potential) and the number of electrons and cation that are reversibly bound (the capacity of a battery) are easily tuned.
In addition to the well-known light-weight high capacity Li-ion batteries for mobile applications, many battery types for stationary applications in which low costs and scalability are more important are under development. This is particularly relevant in the prospect of the current energy transition, for which energy is increasingly generated from intermittent sources like wind and solar. In many stationary applications water is chosen as the electrolyte. This increases safety at the expense of a decreased potential. Examples of stationary batteries under development (with our partners at the RST department) are Na+/water and flow batteries. Organic electroactive materials based on the scaffolds mentioned above can be tuned for these different application by adapting the redox properties and their solubility pattern, as will be illustrate below.
Currently we are developing organic electrode materials for alkali (Li+/Na+) and alkali-earth ion (Mg2+/Ca2+) high capacity batteries for mobile applications. In order to get a decent voltage in the battery, highly electron deficient molecules, such as substituted perylene and naphthalene bisimides (D and C) In these cases a high capacity (based on weight) and multi functionality i.e. combining multiple properties like electron conductivity, electro activity and binding capability as a polymer binder in the same material, are important. Also, for obtaining stable electrodes in Li-ion batteries, the electroactive electrode molecules should not dissolve in the battery electrode material, which in these type of batteries generally is an organic carbonate.
References:
Supramolecular Perylene-Bisimide-Polysulfide Gel Networks as Nanostructured Redox Mediators in Dissolved Polysulfide Lithium-Sulfur Batteries. Frischmann, P. D. et all. Chem. Mater. 2015, 27, 6765−6770.
Naphthalene Diimide Based Materials with Adjustable Redox Potentials: Evaluation for Organic Lithium-Ion Batteries. Vadehra, G. S.; Maloney, R. P.; Garcia-Garibay, M. A.; Dunn, B. Chem. Mater. 2014, 26, 7151−7157.
Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; Sprenkle, V. ACS Energy Lett. 2017, 2, 2187−2204.
Molecular engineering of organic electroactive materials for redox flow batteries. Ding, Y.; Zhang, C.; Zhang, L.; Zhou, Y.; Yu, G. Chem. Soc. Rev., 2018, 47, 69-103.