News
Room temperature synthesis of peryleen diimides (PDIs)
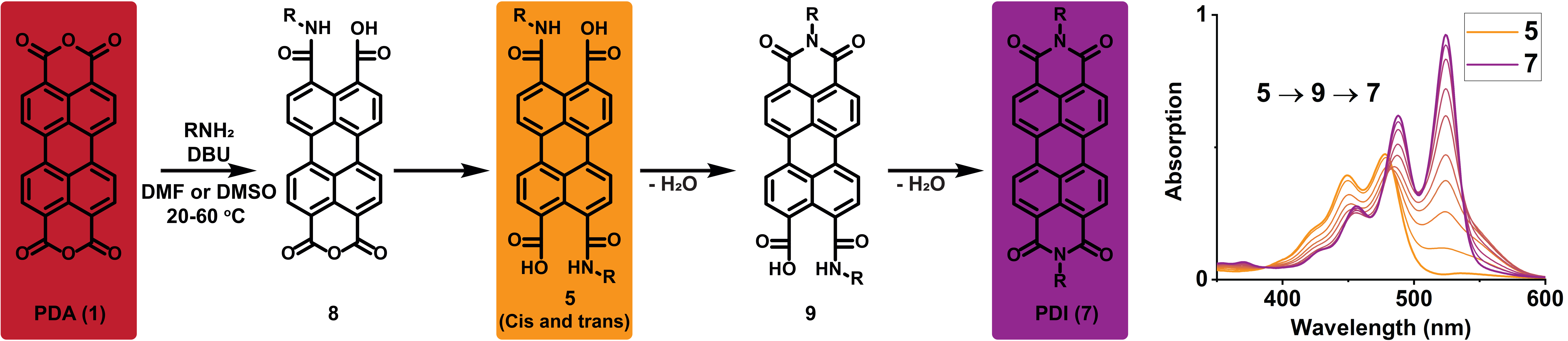
Perylene diimides (PDIs) are an important class of compounds that find commercial applications as dyes and pigments and have high potential for advanced applications in photovoltaics, molecular electronics, artificial photosynthesis, battery materials and photo catalysis. Despite the fact that these compounds are known for more than a century, the synthesis of PDIs from perylene dianhydride (PDA) is harsh and demanding, requiring high temperatures and a protective atmosphere.
In our manuscript “Room temperature synthesis of perylene diimides facilitated by high amic acid solubility” we have demonstrated that this synthesis can be performed at low temperatures, room temperature if needed, in quantitative yields.
It turns out that the first step of the reaction, formation of amic acid 5, is fast and quantitative at room temperature in the solvents that we have used. The second step, imide formation, requires elevated temperatures and is notably slower due to limited solubility of the amid acids 5 and 9. By adding water to the reaction mixture, the solubility of the amic acids is drastically increased and the imide formation smoothly proceeds at room temperature. Because these reactions are quantitative, purification of the poorly soluble PBIs is not required. Filtering after the reaction yields pure product! In addition to the room temperature synthesis, an entirely green PDI synthesis using DMSO as a solvent and K2CO3 as a base has been developed.
Currently we are investigating the scope and limitations of this synthetic protocol (in terms of the reagents used) and have started exploring exciting prospects of this novel synthesis. Labelling of biomolecules and controlling polymerisation reactions are examples of reactions that can be realised using this novel synthetic protocol.
Further reading:
Room temperature synthesis of perylene diimides facilitated by high amic acid solubility
Kwakernaak, M. C.; Koel, M.; van den Berg, P. J. L.; Kelder, E.M.; Jager, W. F.
Org. Chem. Front., 2022, 9, 1090-1108. DOI: 10.1039/d1qo01723c
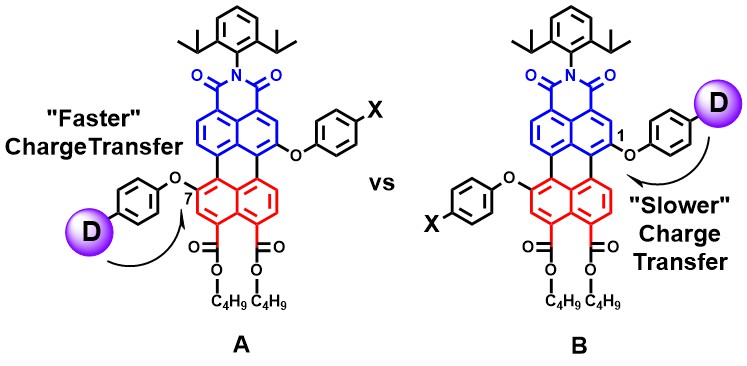
Directing charge transfer in perylene based light-harvesting antenna molecules
Gaining control over charge transfer pathways in photoexcited molecules is a prerequisite for the construction of efficient devices for artificial photosynthesis. The synthesis and photophysical characterization of the regioisomers A and B, enabled us to prove that photoinduced electron transfer (PET) from the electron donor (D) to the perylene acceptor is faster from the 7-position (molecule A) than from the 1-position (molecule B). This result was anticipated because the HOMO of the perylene scaffold along the long-axis is polarized towards the bottom half (red fragment), adjacent to the donor at the 7-position (molecule A). As a result, a slightly better HOMO-HOMO electron coupling is anticipated and a concomitantly faster PET is observed for molecule A, than for molecule B. For the synthesis of compound A, a novel synthetic scheme was developed exploiting anhydride activation of the 7-position, which hitherto has not been explored for the synthesis of perylene chromophores.
Directing charge transfer in perylene based light-harvesting antenna molecules
Abbey M. Philip, Chao Chun Hsu, Zimu Wei, Magnus B. Fridriksson, Ferdinand C. Grozema , and Wolter F. Jager. J. Chem. Phys., 2020, 153, 144302. DOI.
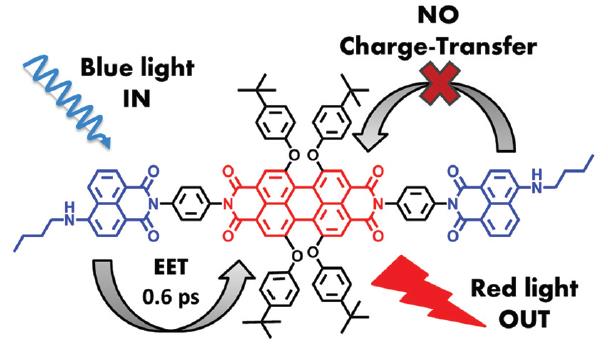
Elimination of Intramolecular Charge Transfer in Perylene Imide Based Light-Harvesting Antenna Molecules.
Light harvesting molecules are an essential component for artificial photosynthesis. In our previous research, using the molecular approach, we have developed antenna molecules composed of naphthylimide (NI) energy donors and perylene tetracarboxylic acid (PTCA) energy donors. These molecules exhibit excellent properties apart from one annoying issue; intramolecular charge transfer between the NI energy and the PTCA energy acceptor in emerges in polar solvents.
Now we have solved this issue simply by placing the NI donors at the peri positions instead of the bay positions. An additional advantage of this approach is that the bay positions, from which photoinduced charge transfer (PET) is known to take place easily, are available for attaching electron donors and acceptors to accomplish the charge separation that is required for artificial photosynthesis.
Efficacious Elimination of Intramolecular Charge Transfer in Perylene Imide Based Light-Harvesting Antenna Molecules. Rajeev K. Dubey, Damla Inan, Abbey M. Philip, Ferdinand C. Grozema and Wolter F. Jager. Chem. Commun, 2020, DOI.
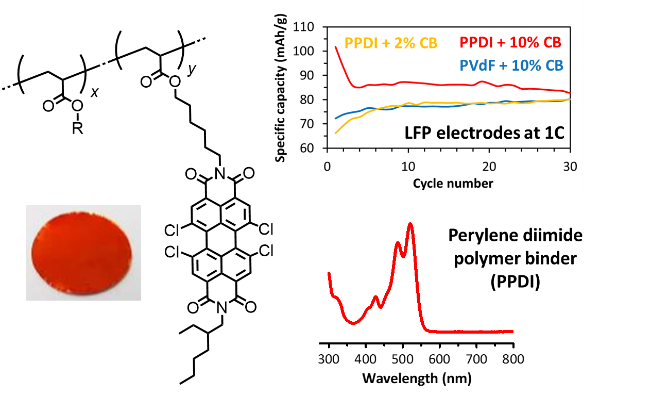
PBI containing side chain polymers as Li-ion battery electrode materials
Electrodes in lithium ion batteries are mainly composed of electroactive materials, materials that reversibly get reduced, taking up electrons and Li ions. Also electron conductive materials, materials that conduct electrons, and polymer binders, materials that keep all components in close contact, are added. Commercial Li-ion batteries contain inorganic electroactive materials, but in this research we have replaced them by organic materials; perylene bisimides attached to a side-chain polymer. Advantage of this organic approach is flexibility; redox levels are easily tuned and many opportunities exist for attachment to polymers. It turns out that the polymer that we have developed has excellent electrochemical stability, exhibits electron conductivity and also acts as a polymer binder.
Further reading:
Scalable route to Electroactive and Light active Perylene diimide dye polymer Binder for Li ion Batteries.
Ranque, P.; George, C.; Dubey, R. K.; van der Jagt, R.; Flahaut, D,; Dedryvère, R.; Fehse, M.; Kassanos, P.; Jager, W. F.; Sudhölter, E. J. R.; Kelder, E. M.
ACS Appl. Energy Mater., DOI
How many substituents can the perylene scaffold bear?
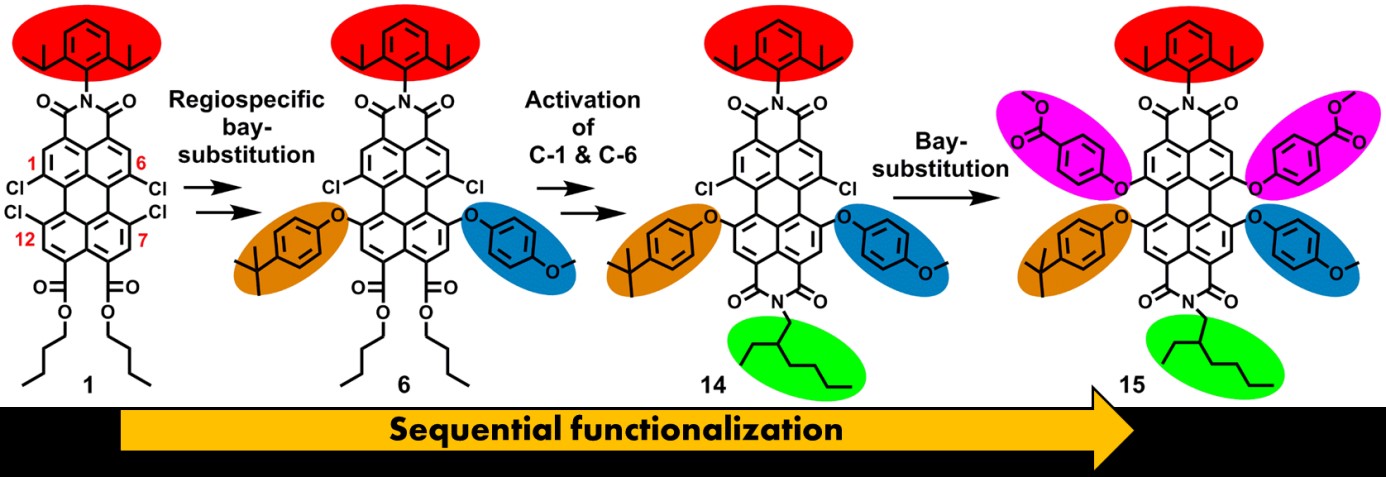
Science is often compared with sports and in this Olympic Year we know that sports is very much about breaking records. In science, synthetic chemistry in our case, bringing the discipline to a higher level, is often substantiated by breaking records as well. One such record is the number of independent substituents one can attach to the perylene scaffold. The theoretical limit is 12, but clearly nobody has ever been close to that. In our manuscript “Synthesis of Perylene-3,4,9,10-Tetracarboxylic Acid Derivatives Bearing Four Different Substituents at the Perylene Core”, DOI, that was published 4 years ago we set the record on 4, and now we have raised the bar higher; five independent substituents attached to a PDI. This was possible by exploiting the pronounced difference in activation of the chlorine atoms in the starting compound tetrachloro monoimide diester (1). First, the chlorine atoms at the highly reactive imide-activated positions 7 and 12 are substituted subsequently. After transforming the peri esters to a imide functionality, the imide activated chlorine atoms at positions 1 and 6 are substituted, and compound 15 is obtained.
So far, the work described yields model compounds, providing proof of concept. However, for advanced applications, such as artificial photosynthesis the precise placement of multiple functional groups, energy donors for the antenna function, and electron donors and acceptors for charge, is an absolute requirement.
With a smart choice of substituents attaching the sixth substituent appears to be within reach, but for a further extension it is required to also bring the ortho substituents in the game. In view of the recently developed chemistry for attaching ortho substituents, this is a realistic option.
Further reading:
Perylene Bisimide Dyes with up to Five Independently Introduced Substituents: Controlling the Functionalization Pattern and Photophysical Properties Using Regiospecific Bay Substitution
Dubey, R. K.; Eustace, S. J.; van Mullem, J. S.; Sudhölter, E. J. R.; Grozema, F. C.; Jager, W. F.
J. Org. Chem. 2019, 84, 9532−9547 DOI