New 3D co-culture model to study the effect of proton therapy on aggressive brain cancer
Researchers at TU Delft designed novel 3D-engineered scaffolds inspired by the geometry of the brain microvasculature. The micro-structures were co-cultured with both glioblastoma, an aggressive brain cancer, and endothelial cells, the building blocks of blood vessels. This model enabled researchers to study the effect of proton therapy on glioblastoma and uncovered a possible protective role of endothelial cells on cancer cells.
“Tumours are mostly studied in 2D environments (e.g. petri dishes) or via animal experimentation. Since these models lie miles apart, it is very difficult to translate findings from 2D-models to the human context,” says Angelo Accardo, assistant professor at the Department of Precision and Microsystems Engineering. “Besides, there are obvious ethical implications related to the use of laboratory animals.” Accardo aims to bridge the gap of in vitro (cells) and in vivo (living organism) models. Together with his PhD student Qais Akolawala and collaborators from Holland Proton Therapy Center (HollandPTC) and LUMC, Accardo developed 3D-engineered scaffolds that resemble the microvascular architecture of our brain, contributing to a better understanding of how proton therapy affects glioblastoma.
Minimising collateral damage
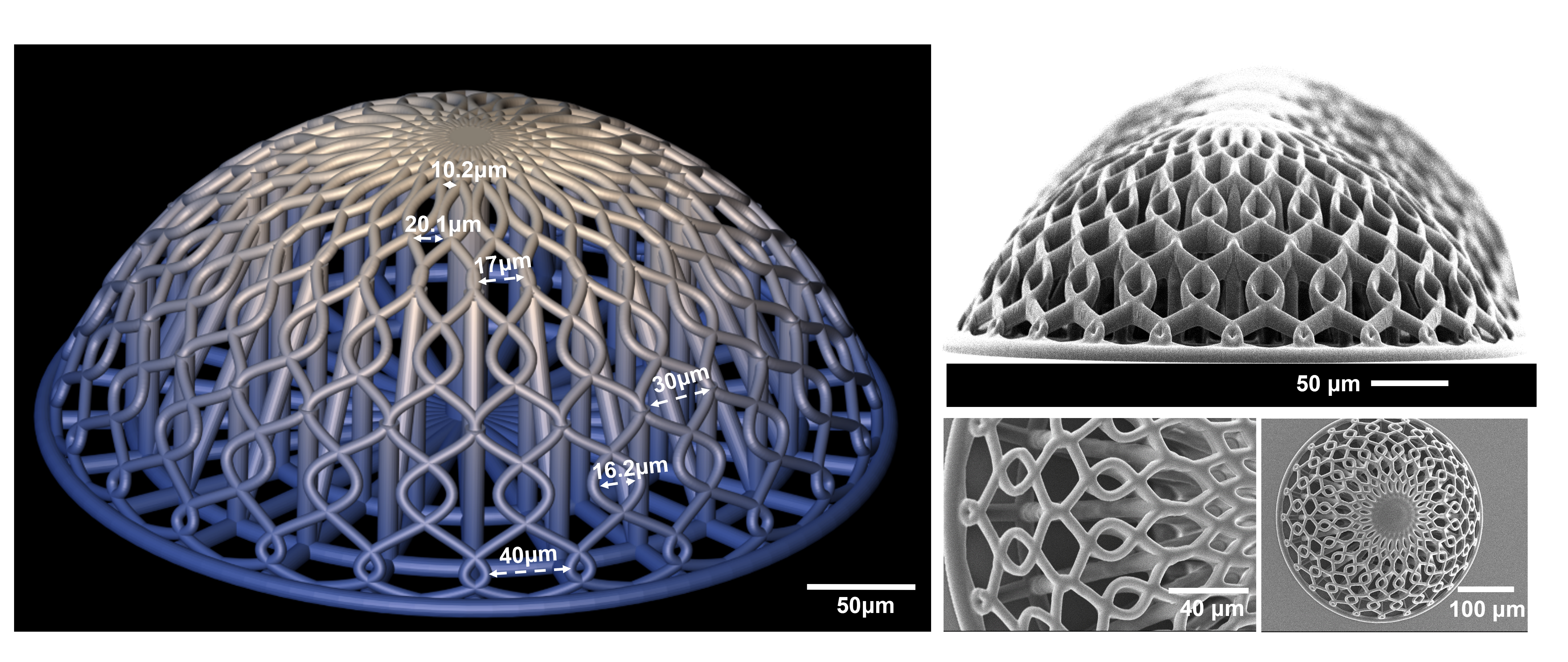
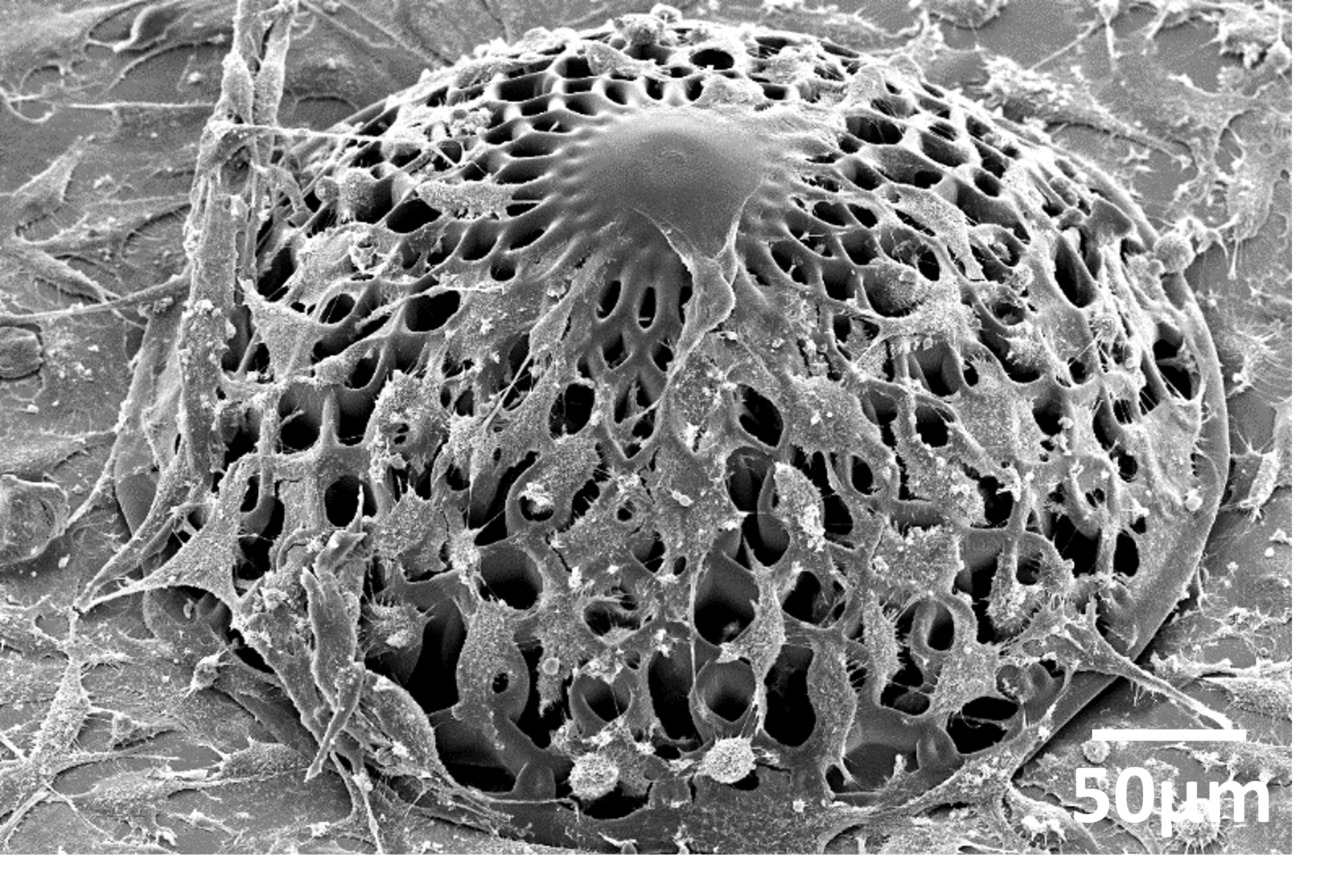
It has been shown that cells behave and respond to their external environment, and specifically the shape of their environment. “To create a biologically relevant model, we need our 3D-engineered scaffolds to mimic the natural environment as much as possible. Since glioblastoma cluster and proliferate around blood vessels in the brain, we designed tiny vessels and curvature in the model,” explains Akolawala. They fabricated these engineered microenvironments with the micromanufacturing technology known as two-photon polymerization. First, the researchers cultured the cells that line blood vessels, endothelial cells, on the manufactured microstructures, followed by human malignant glioblastoma cells.
Glioblastoma is a very aggressive form of cancer with a poor prognosis. While surgery, chemotherapy and X-ray radiotherapy remain the current standard, proton beam therapy is an appealing alternative as protons can damage cancer cells while sparing the surrounding healthy tissue. Accardo: “This is because protons are able to release their energy, that causes cell damage, at a specific point within the patient’s body.” Since proton therapy is a relatively newer technique compared to X-rays, there’s a need for a better understanding of proton-cell interaction, within a physiologically relevant microenvironment, and how much it actually damages cancer cells.
An “engineered” benchmark tool for proton radiobiology
The new 3D model can contribute to this. “We observed that the DNA damage induced by protons to glioblastoma cells in our 3D model is much more similar to the damage reported in in vivo models compared to 2D models,” says Accardo. In addition, glioblastoma cells cultured with endothelial cells featured less DNA damage than the model with solely glioblastoma cells, indicating that endothelial cells play a role in the radio-resistance of glioblastoma cells. “We hypothesize that either endothelial cells have a protective role on glioblastoma cells via biochemical interaction, or that endothelial cells increase the stemness of brain cancer cells which affects their level of aggressivity,” says Accardo.
The study, published in Advanced Healthcare Materials, showed that the 3D-model is physiologically relevant and reproducible. “Our next step would be to extend our approach to patient derived cells and test other modalities of proton radiation (e.g. FLASH).” Accardo’s ultimate goal is to employ this 3D-model to examine the response to radiation of patient derived cells, paving the way for the realization of personalised treatment strategies.