3mE student wins prize for best master thesis at the Energy Challenge Event
On June 9, the TU Delft campus was buzzing with students' ideas about the future of energy transition. All faculties nominated their most appealing course from the faculty to the Energy Challenge Event to present their solution for the energy transition. Of all master students, Bart Boons won the prize with his master thesis about system integration of a high pressure alkaline electrolyser.
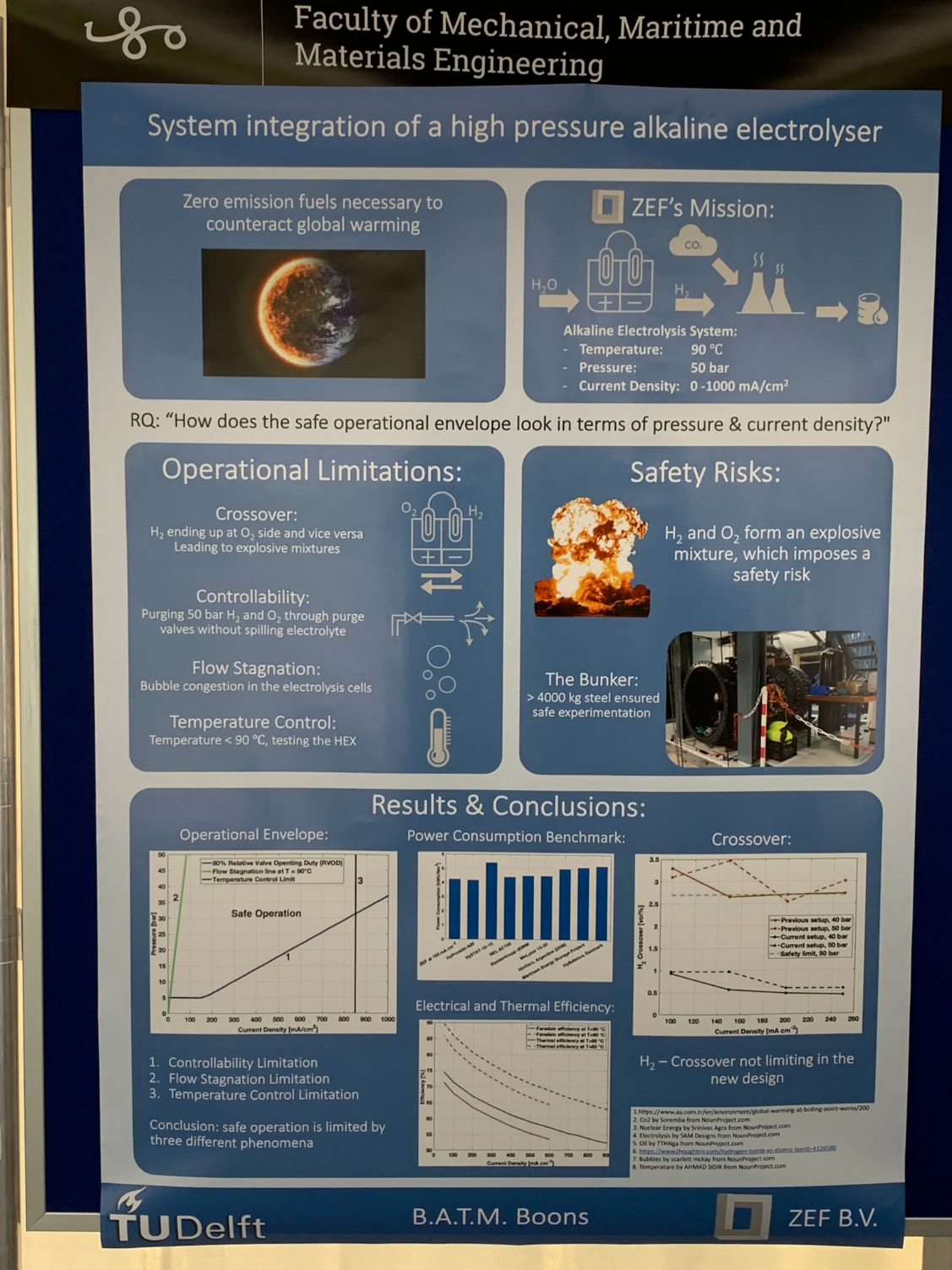
Bart Boons recently graduated in Mechanical Engineering: Energy, Flow & Process Technology. He has worked on the improvement in efficiency and enlargement of the operation envelope of a ‘high pressure alkaline electrolyser’. The electrolyser under concern is a crucial component in a ‘stand-alone’ energy conversion system, that air-captures carbon dioxide and water, and converts this based on solar power solely into methanol as a versatile energy carrier. The system is being developed by ZEF, a start-up company run by alumni with their lab-facilities housed within 3mE’s Process & Energy department and in close cooperation with P&E staff members and students.
Electrolysis process
Electrolysis of water into Hydrogen (H2) and Oxygen (O2) is simple and demonstrated in any high-school chemistry class. So is usually demonstrated that hydrogen can be dangerous when mixed with oxygen. In industrially relevant continuous operation electrolysers, there is always the issue of safe control of the system operating at a high conversion efficiency, which is obtained through a continuous recirculating flow of the alkaline water along the electrodes, carrying away the two formed gaseous products with a lowest level of mixing of the two. Furthermore, the gases need a controlled release from the system – for further processing.
New control strategy
The contribution of Bart is that he has developed a new control strategy for the system: he has proposed the method and set up a model, done dynamic simulations for the system behaviour, and done validation experiments by physically building the system and testing it on an existing electrolyser. The results are promising and show that the proposed model may indeed improve system performance. Yet the experiments also show that the real world – with real tubes, valves and vessels, and with real flow fields with bubbles – is more complex than a model world, and that on various points theory is over-optimistic.