Hydrogen is a versatile energy carrier that may help significantly reduce greenhouse gas emissions, thereby playing an important role in the energy transition. It being highly flammable and even explosive under certain conditions, however, means that even small leaks must be quickly detected for safe use. Lars Bannenberg and his colleagues develop materials for cheap, reliable and small hydrogen sensors.
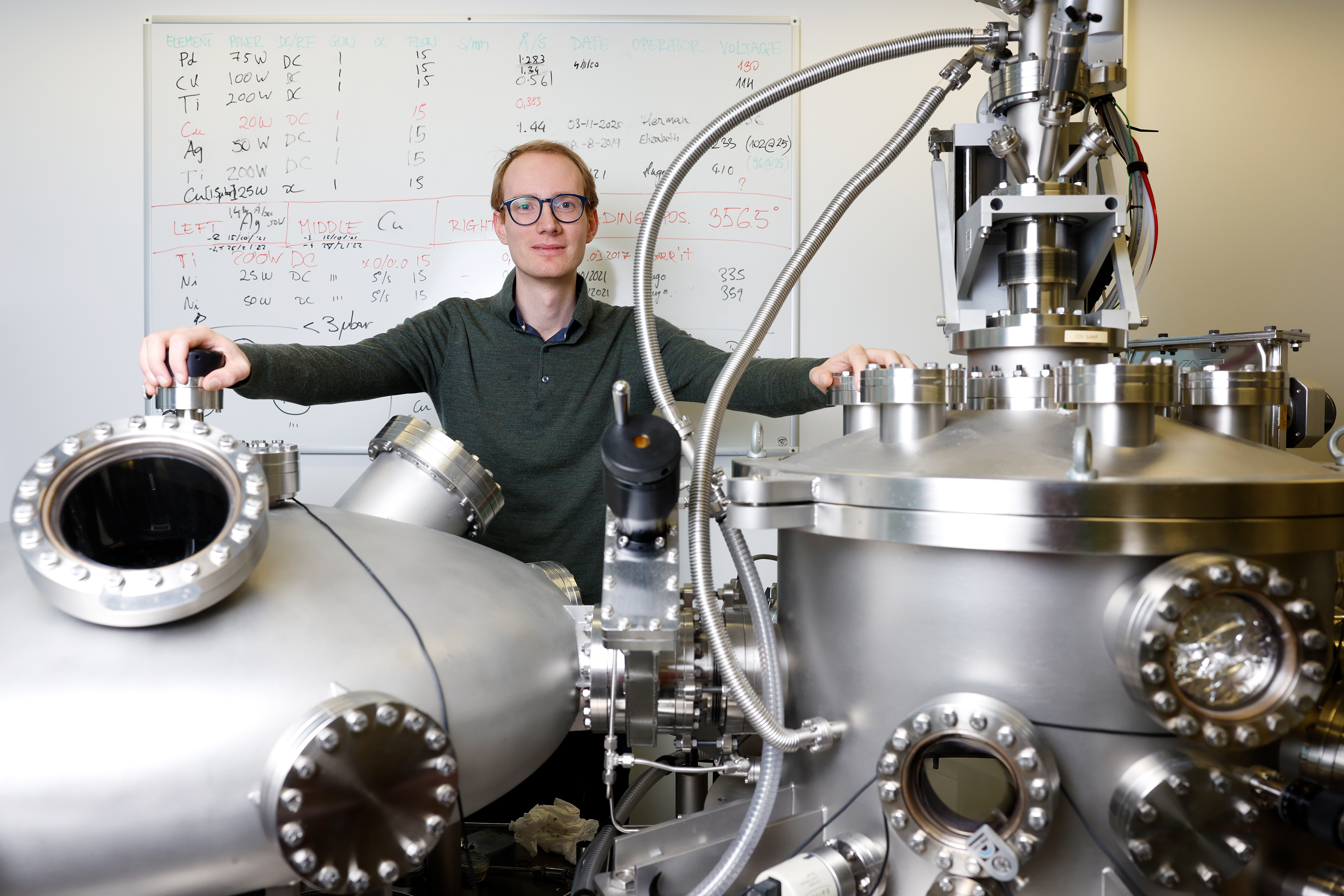
“I never work alone, but always collaborate with other people,” Lars Bannenberg stresses several times during our interview. It takes place in an office, but it is clear from all he does and says that he feels very much at home in a lab, surrounded by experimental setups. Soon after completing his highly fundamental PhD research (into skyrmions and chiral magnets), he decided to set out on doing something with more immediate societal relevance. “Reducing greenhouse gasses, by using hydrogen as an energy carrier, to me is one way to establish a fully sustainable economy. And for mankind to make it into the 22nd century.”
Using hydrogen as an energy carrier, to me is one way to establish a fully sustainable economy, and to have mankind make it into the 22nd century.
Lars Bannenberg
For many years, the faculty of Applied Sciences, within which Lars is an assistant professor, has hosted research into metal hydrides. These are formed when metals such as palladium and tantalum absorb hydrogen. In turn, this may alter certain physical properties of the metal, such as how well it will reflect or transmit light. “For quite some time, we had the idea of using this property to develop a highly sensitive and widely applicable optical hydrogen sensor (see frame),” Lars says. “When the COVID-19 pandemic left many laboratories and experimental setups unused, my colleagues (such as Herman Schreuders and Bernard Dam) and I set out on some initial experiments.”
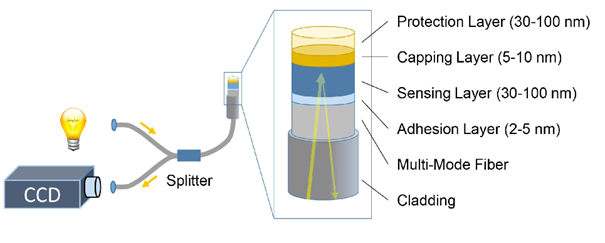
To increase the efficiency of their intended sensor material, they separated two important functions over two very thin layers. “It gave us much more room to manoeuvre,” Lars says. “The sensor material consists of a thin-film layer of a tantalum alloy that creates the optical contrast. It is covered with a catalytic layer that dissociates the molecular hydrogen. Thanks to this separation, our sensor has a response time of less than a second.”
The properties of thin-film materials may differ from that of bulk material. We use this to suppress certain unwanted material properties.
Lars Bannenberg
They were quite surprised to see their first experiment yield impressive results, but it certainly wasn’t an accidental finding. “We designed our sensor material using the knowledge available within our research group,” Lars says. “For one, we make good use of the fact that the physical properties of thin-film materials may differ from that of bulk material. We use this to suppress certain unwanted material properties.”
They also immediately were able to sense hydrogen concentrations spanning seven orders of magnitude – corresponding to a kitchen scale measuring anything from a few grams to the weight of an elephant. It is a huge and important improvement over currently available hydrogen sensors as there are situations in which you want to be able to measure tiny concentrations (see frame). “Our detection range probably exceeds these seven orders of magnitude, but it is beyond the range of our laboratory equipment,” he proudly adds.
The initial experiments were followed by many more experiments to fully understand why the sensor material is so effective and to enable further optimisation. These experiments involved many of the measuring techniques and experimental setups Lars used during his PhD and master’s thesis work. One of these is the neutron reflectometer at the TU Delft Reactor Institute, of which Lars is the instrument scientist. “With neutrons you can measure tiny amounts of hydrogen inside a material. But we also used X-rays to understand how absorbed hydrogen alters the structure (crystal lattice) of the material.”
Current hydrogen sensors are relatively large (the size of a smoke detector) and expensive (costing hundreds of euros). The new material offers many advantages. “We deposit the sensing layer on top of an optical fibre that is less than 0.2 millimetre in diameter,” Lars says. “And even though palladium is very expensive, the thin-film layer consists of only a few cents worth. A complete sensor, including read-out electronics, will obviously cost more than that.” Lars currently has an electrical engineering master’s student working on a prototype. “It is crucial that the full sensor retains all its advantages.”
The new sensor is also inherently safe as, for read-out, it doesn’t require the presence of oxygen or an electrical current close to the sensing material – something that normally brings the risk of ignition. And contrary to current sensors, the new sensor doesn’t require calibration at regular intervals. Lars: “From a safety perspective, it doesn’t help if a detector is expensive and requires calibration.” Further research may also the new sensing material fit for use in fuel cells and electrolysers, allowing the more efficient and thereby cheaper conversion of electricity to hydrogen and vice versa.
Our sensing material is less than a millimetre in diameter, inherently safe, very precise and costs only a few cents.
Lars Bannenberg
The group is pleased to have been awarded patents for an entire range of sensor materials. In the meantime, Lars and his colleagues continue working on several applications. “We are currently developing sensors for hydrogen-powered aircraft, together with researchers from the faculty of Aerospace Engineering,” he says. A major challenge is to have the sensor function properly at both very low temperatures and very high temperatures (from -60 to 250 degrees Celsius). “The aviation industry prefers to have a single sensor as everything must be certified. It’s a nice puzzle.”
There are more sectors that have shown interest in collaboration and commercialisation. Launching a start-up is also an option, one that fits nicely with the master’s degree in economics and business economics that Lars obtained in parallel to his physics degree. If so, it will be a side project, as he thoroughly enjoys his academic freedom at TU Delft as well as the questions that will come from further research. “In particular: formulating those questions!”
More information:
Delft researchers develop a versatile hydrogen sensor (tudelft.nl)
Three applications of the new hydrogen sensors
Safety: A mix of only 4% hydrogen, and therefore 96% air, is explosive. One must be able to detect much lower concentrations.
Climate: Hydrogen in the air increases the lifetime of methane, a very potent greenhouse gas. You want to detect leaks with low hydrogen concentration (and a large flow).
Process optimisation: For example for the more efficient conversion of hydrogen to electricity in fuel cells.