Nanomaterials for hydrogen production
Ruud van Ommen is developing techniques to produce nanomaterials on an industrial scale. Expensive precious metals that serve as catalysts are the bottleneck for scaling up water electrolysis.
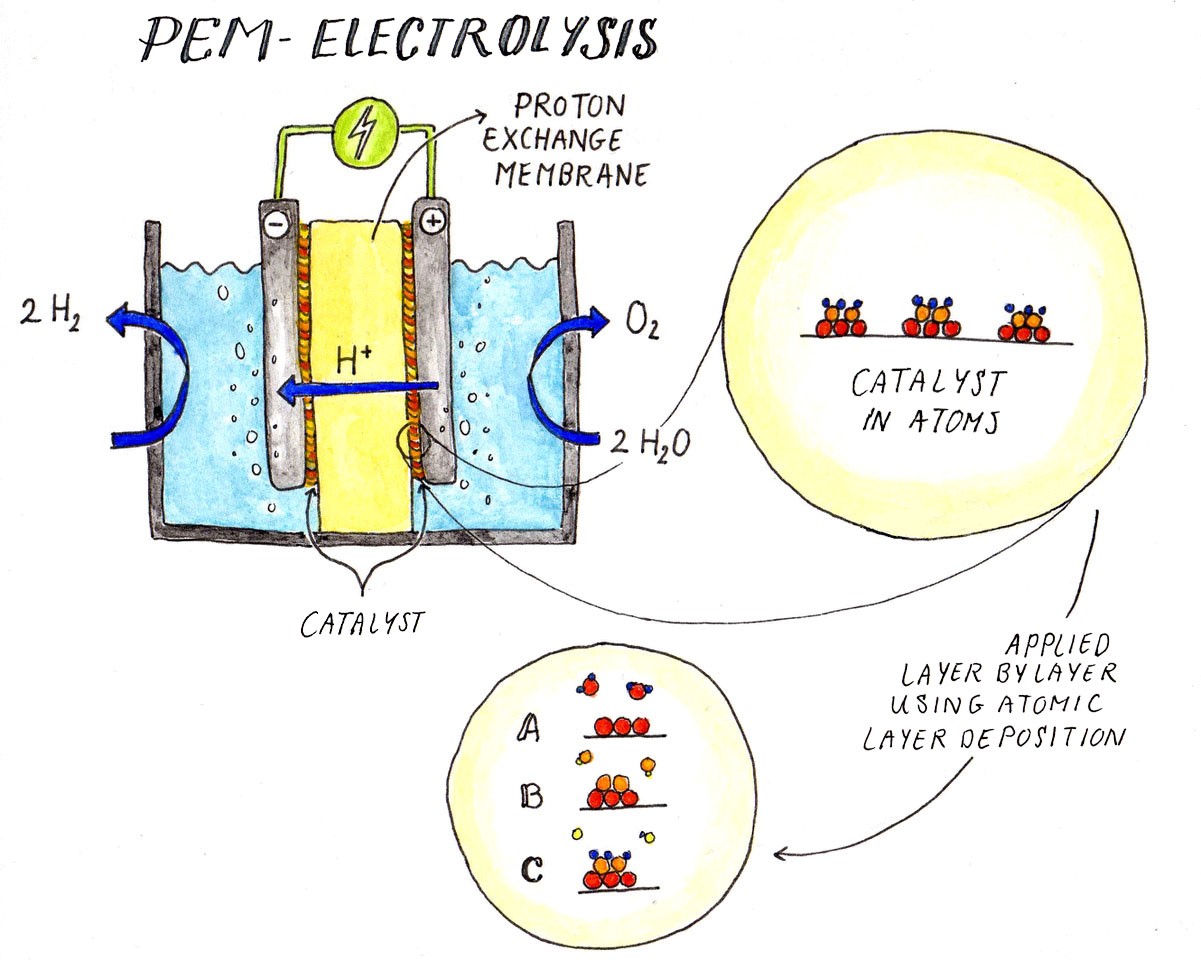
Roughly speaking, there are two types of water electrolysis,” explains chemical technologist Ruud van Ommen of the Faculty of Applied Sciences. “Alkaline electrolysis, a relatively old technology developed by the industry, and Polymer Electrolyte Membrane (PEM) electrolysis.” The latter technique separates hydrogen and oxygen directly through a proton exchange membrane and looks to be the most promising method. Van Ommen: “The challenge with this technique is that it requires expensive precious metals to act as a catalyst.”
Roughly speaking, there are two types of water electrolysis,” explains chemical technologist Ruud van Ommen of the Faculty of Applied Sciences. “Alkaline electrolysis, a relatively old technology developed by the industry, and Polymer Electrolyte Membrane (PEM) electrolysis.” The latter technique separates hydrogen and oxygen directly through a proton exchange membrane and looks to be the most promising method. Van Ommen: “The challenge with this technique is that it requires expensive precious metals to act as a catalyst.”
Water electrolysis has an efficiency of between 60-75 and maximum 80%. “This efficiency has everything to do with the current density,” explains renewable energy storage researcher Hans Geerlings (Applied Sciences). “If you force the electricity to flow slowly the efficiency increases; if you give the electrolyser a boost the conversion becomes less efficient. This must be factored in if you want to scale up.” According to Van Ommen, there is little to be gained by increasing the efficiency of PEM electrolysis. “The key is to significantly reduce the amount of the catalyst iridium. We currently need 200 mg of iridium per kW, which must be reduced by at least a factor of 20.”
Small clusters of atoms
Van Ommen is working on this step using Atomic Layer Deposition (ALD), a technique from the chip industry. The atoms of the catalyst are introduced in clusters, which increases the surface area and so requires less catalyst. This technique also works for catalysts of other energy applications, such as platinum in hydrogen cars and cobalt in electric cars.
Van Ommen’s research has resulted in a spin-out company, Delft IMP (Intensified Materials Production). Delft IMP produces nanomaterials using ALD on a kilogram scale. The technology is still under development, but Van Ommen expects IMP to produce nanomaterials on a tonne scale within a few years. Geerlings expects water electrolysers to be scaled up quickly too. “Solar and wind energy are on the rise, and that requires good electrolysers. Within ten years water electrolysis will be carried out on a large scale worldwide.”