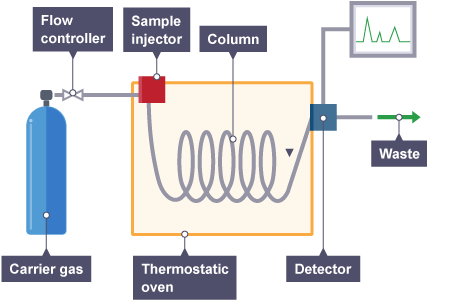
Gas chromatography (GC)
In gas chromatography, the mobile phase is a carrier gas, usually an inert gas such as helium. The stationary phase is a microscopic layer of liquid or polymer on an inert solid support, inside a piece of glass or metal tubing called a column. Hence the full name of the procedure is "Gas–liquid chromatography", referring to the mobile and stationary phases, respectively.
(GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). One of our GC is used to quantified the amount of VFA (volatized fatty acid) or the compositions of the biogas.

The gaseous (or to be evaporated liquid) compounds being analyzed interact with the walls of the column, which is coated with a stationary phase. Due to interactions degrees taking place between sample the components and the adsorbent, this causes each compound to be elute at a different time, known as the retention time of the compound.
Gas chromatography is in principle similar to HPLC, but has several notable differences. First, the process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas mobile phase, whereas in column chromatography the stationary phase is a solid and the mobile phase is a liquid. Hence the full name of the procedure is "Gas–liquid chromatography", referring to the mobile and stationary phases, respectively. Second, the column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled exactly. Finally, the concentration