Organochlorides Mediate Oxidation Reactions Induced by Low Dose Ionizing Radiation
In cancer treatment, there exists an acute need to deliver drugs exclusively at the tumor site. This necessity arises from the adverse side effects associated with systemic treatments and the potential utilization of highly cytotoxic drugs. The use of ionizing radiation to trigger the release of chemotherapeutic drugs holds great promise in this regard, both to achieve local, targeted release as well as in the context of combined radiotherapy-chemotherapy approaches.
However, a substantial challenge persists across these methodologies: the inherent insensitivity of molecular processes to ionizing radiation at clinically relevant radiation doses. In simpler terms, inducing selective breaking or making of chemical bonds -essential for drug release or activation- is exceedingly difficult at low radiation doses. To date, only a handful of highly specific methods have been reported.
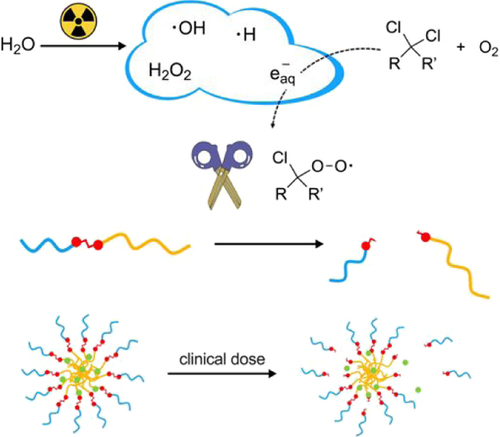
In a collaborative project with prof. Antonia Denkova (RST), where we discovered that certain organochloride compounds significantly amplify radiation-induced oxidation, this can be used to initiate release and activation of drugs. Remarkably, our method already demonstrates efficacy with a mere 4 Gy dose of gamma or X-rays, well within the confines of standard clinical radiotherapy doses.
J. Liu, T. G. Brevé, B. Xu, P.-L. Hagedoorn, A.G. Denkova, R. Eelkema
Abstract
The controlled release of drugs using local ionizing radiation presents a promising approach for targeted cancer treatment, particularly when applied in concurrent radio-chemotherapy. In these approaches, radiation-generated reactive species often play an important role. However, the reactive species that can be used to trigger release have low yield and lack selectivity. Here, we demonstrate the generation of highly oxidative species when aqueous solutions containing low concentrations of organochlorides (such as chloroform) are irradiated with ionizing radiation at therapeutically relevant doses. These reactive species were identified as peroxyl radicals, which formed in a reaction cascade between organochlorides and aqueous electrons. We employed stilbene-based probes to investigate the oxidation process, showing double bond oxidation and cleavage. To translate this reactivity into a radiation-sensitive material, we have synthesized a micelle forming amphiphilic block copolymer that has stilbene as the linker between two blocks. Upon exposure to ionizing radiation, the oxidation of stilbene led to the cleavage of the polymer, which induces the dissociation of the block-copolymer micelles and the release of loaded drugs.