No constants for catalysis
Nieuws
-
10 mei 2022
-
Communication ChemE
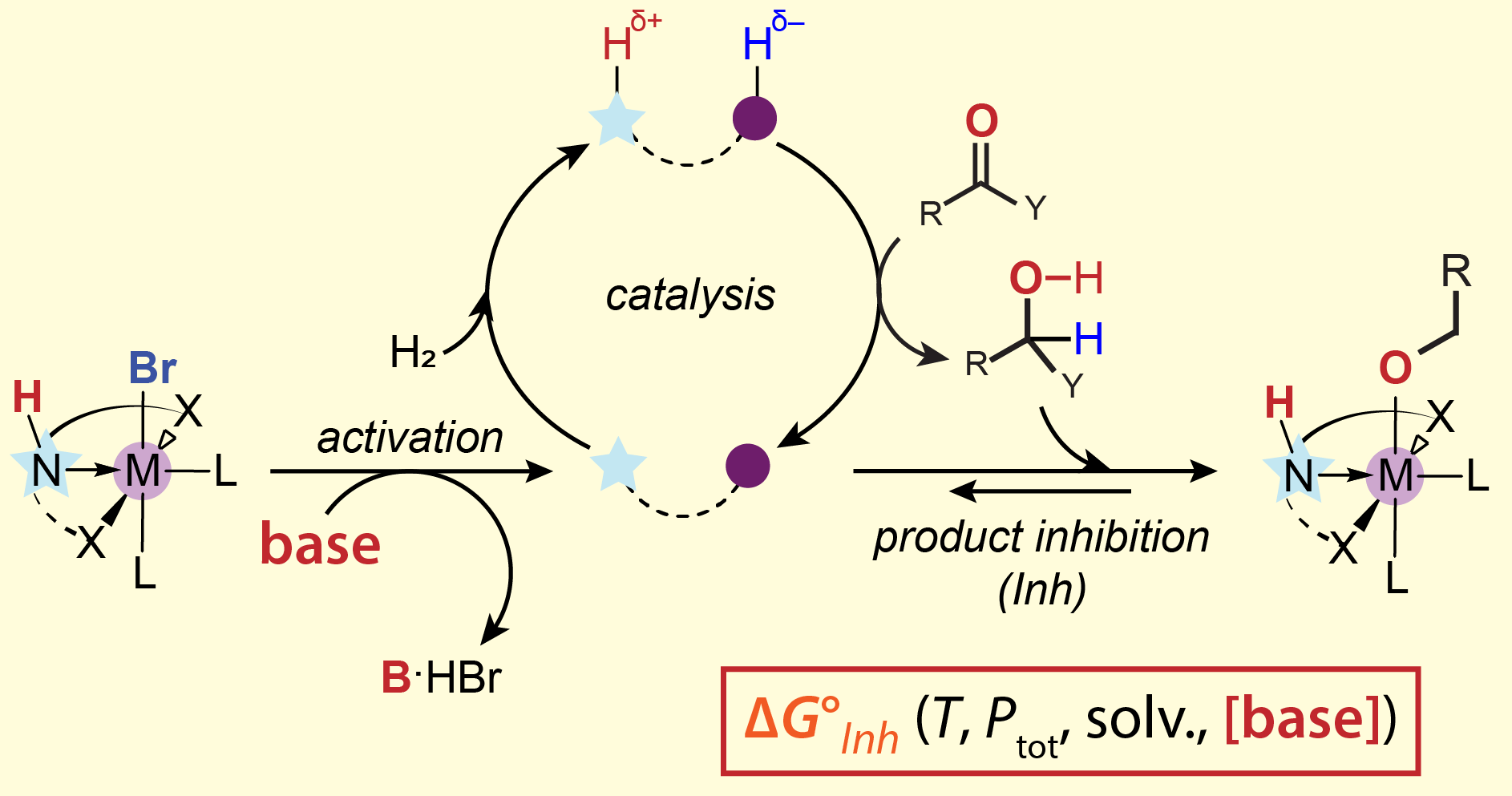
We view chemical reactions through the lens of kinetic and thermodynamic descriptors. Whether it’s a simple equilibria or a complex catalytic reaction – a set of constants and a correct model is expected to describe the behavior of the chemical system. The work of PhD candidate Wenjun Yang (ChemE) working with professor Evgeny Pidko suggests that reality is more complex.
In a recent issue of the Journal of the American Chemical Society they show that the state of the catalyst and even standard thermodynamic parameters, long thought to be ironclad constants, are a function of condition space – solvents, concentrations and reaction promoters. Read more about this work online.