Limits of Defect Tolerance in Perovskite Nanochrystals
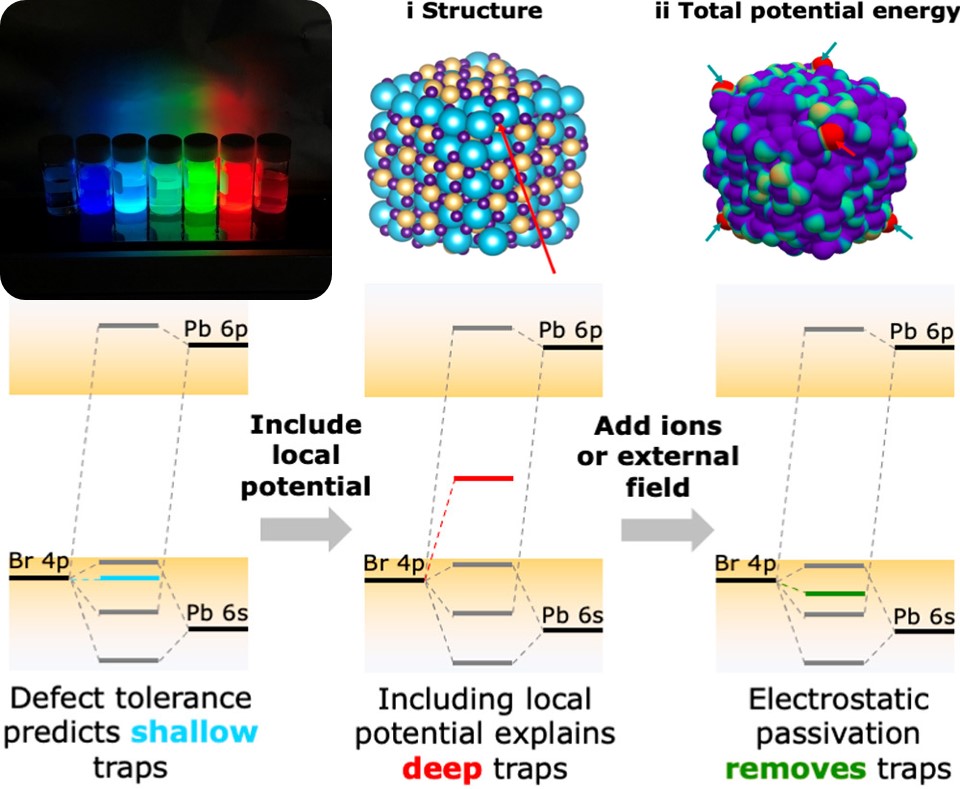
Lead halide perovskites are a peculiar class of semiconductors. Perovskite solar cells have skyrocketed to efficiencies of 26% in a few years. Perovskite nanocrystals can have photoluminescence quantum yields of 100%, making them highly promising for applications in e.g. lighting and lasing. All this is usually explained by the “defect tolerance” of the perovskite electronic structure: as a result of the specific atomic orbitals that join to form the conduction and valence bands, structural defects or undercoordinated surface ions should not lead to electronic states in the bandgap, so-called “traps”, which usually lead to all sorts of trouble. And yet, both theoretical and experimental studies show that undercoordinated halide ions on the surface do sometimes form traps.
Indy du Fossé and coworkers from the NCFun group of prof. Arjan Houtepen have shown that the traditional picture of defect tolerance is incomplete and should also include the local electrostatic environment.
In most semiconductor materials, undercoordinated atoms lead to localized electronic states that lie somewhere in the bandgap. These trap states cause non-radiative recombination of electrons and holes and thus lower the efficiency of a device you build with these materials. Lead halide perovskites, like CsPbBr3, however, behave differently because of their defect tolerance. Due to the electronic structure of the valence and conduction band, the traps from undercoordinated atoms are expected to lie outside the bandgap, making them a whole lot less troublesome. However, various studies have shown that undercoordinated bromide atoms still lead to traps inside the bandgap, which cannot be explained by the traditional picture of defect tolerance.
In their recent publication in the Journal of the American Chemical Society, Du Fossé and colleagues explain this discrepancy by showing that you should also take the local electrostatic potential into account. An undercoordinated bromide at the surface of the nanocrystal can experience a different potential than a bromide somewhere in the middle of the particle. This potential can push the traps related to the surface bromide from inside the valence band all the way up into the bandgap. Not only do these findings increase our understanding of traps in lead halide perovskites, they also point the way to new methods for removing these unwanted traps. Usually, one tries to covalently bind another molecule (a “ligand”) to an undercoordinated bromide to remove the trap state. Du Fossé and coworkers show that simply adding a positive ion near the bromide also does the trick. It lowers the electrostatic potential around the bromide and thus pushes the trap out of the bandgap.