Chocolate letters, Christmas tree ornaments and the very familiar ice-cream cake with crunch layers: for many people chocolate is inextricably linked to the holiday season. Hardly anyone realises that making creamy, soft chocolate has much in common with making super-strong steel.
Humanity has been using steel for thousands of years, but until recently its production was a question of trial and error. ‘A swordsmith in the Middle Ages didn’t know what we know today. If a sword came out right, then it was viewed as a “magical” sword; the rest could be thrown back into the melting pot,’ says Jilt Sietsma, professor of microstructure control in metals. ‘A good smith was therefore a key player in a city’s safety,’ adds Marcel Sluiter, associate professor of computational materials science. ‘Two discoveries in the late nineteenth century gave our understanding of steel a huge impetus: the electron and X-radiation. Today, X-radiation techniques and electron microscopes enable us to examine the structure of materials in minute detail; the electron is crucial to this structure,’ says Sietsma.
A key observation that could be made as a result is that steel undergoes phase transitions within a solid state. We understand phases in daily life as the transition of a liquid to a solid or a gas, or vice versa, but when a solid undergoes a phase transition the atoms arrange themselves in a different crystal structure. ‘Steel contains 96% iron by weight and half a per cent carbon by weight. The crystal structure is stable at room temperature, but if you heat it up, the way the carbon atoms are arranged in the iron changes, and at a slightly larger scale, the microstructure, an enormous collection of crystals start to form,’ says Sietsma. It’s these microstructures that are responsible for the properties of steel.
To influence these microstructures, steel is heated to 900 degrees, or what’s referred to as the gamma phase, but we use steel at room temperature, so it’s cooled down again. ‘Atoms are always moving towards a steady state. In decreasing temperatures they can move increasingly slowly; so it’s all about how much time we give the atoms,’ he explains. ‘The trick is to not allow them to reach a steady state but something in between, so you can achieve a microstructure that’s between the one condition and the other. You interrupt that transitional process by cooling down quickly to room temperature.’
Persen en walsen
The different microstructures don’t only emerge as a result of temperature changes but also mechanical treatments such as pressing or rolling. ‘By alternating these treatments during production you can create many different properties and thus increase the strength, for example, by a factor of ten. That happens at an extremely large scale, in which sheet metal undergoes a specifically selected temperature-time profile,’ Sluiter explains.
Strength vs. ductility
Two important properties of steel are its strength and the degree to which it can be deformed, the ductility. ‘Depending on the application, you strike a balance between strength and ductility. For example, you want the crumple zone of a car to absorb the force of a collision, so that material has to be as deformable as possible.’ The microstructure plays a role here too, because large crystals are more deformable but less strong, while small crystals are strong but less deformable. One of the challenges of the research is to increase both the strength and the elongation of steel, for example to produce lighter cars.
Materials science
That the microstructure leads to certain properties is not only true of steel: it’s the central theme in materials science. ‘Steel is really special, because of the phase transition in the solid state, which creates so many possibilities for variation in the properties. But in glass, plastic and concrete the microstructures also has an impact on the properties. It’s the same for all materials,’ says Sluiter. ‘Those properties are the result of the chosen composition, the subsequent treatment and the resulting microstructure,’ Sietsma explains. ‘For the development of materials, you usually reason the other way around. Depending on the desired properties, you examine which microstructure you need and how to achieve it.’
Chocolate
Just like in materials science, microstructures play a major role in nutritional research, where they influence properties such as taste and shelf life. There are striking similarities between steel and chocolate: ‘Temperature change, phase transition, deformation: chocolate is comparable in all these areas,’ says Sluiter. ‘The atoms in chocolate seek equilibrium. That, in turn, depends on the composition, the temperature and the way that you deform. It’s also true for chocolate that the equilibrium structure is not the structure that you’re looking for. If that was the case, then it would be a lot easier to produce.’
Phase five
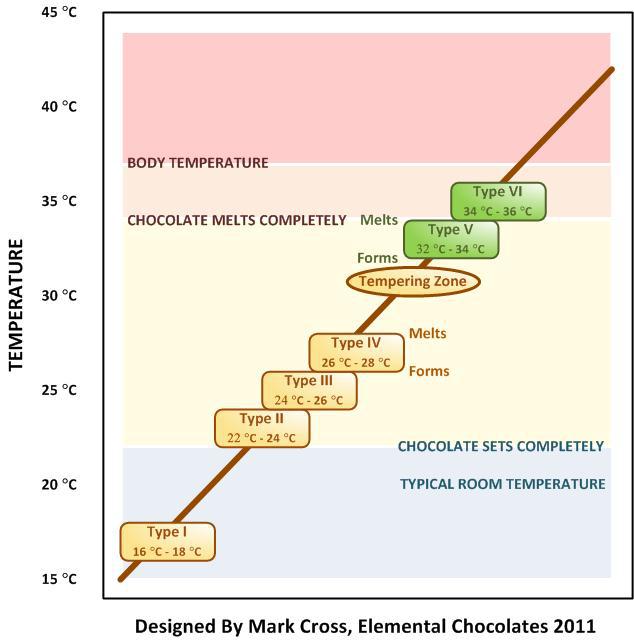
Deforming chocolate is called conching, or rolling, just like with steel. It takes place during the mixing of cocoa, cocoa butter, sugar, milk powder and other ingredients. Then comes the tempering. ‘Treating the chocolate with temperature is extremely important, just like with steel, because that’s when the microstructure is formed,’ Sluiter explains. Chocolate is a complex product that can have six different crystal forms. ‘Crystal form five is the one that the chocolatier aims for. It has a melting point of 32 to 34 degrees. The temperature variations between the different forms are very close to each other, however. If the setting, the cooling off, does not occur properly, then you’ll end up in the wrong chocolate phase. Then it will melt too quickly or not at all. That won’t do the eating experience any good.’
Chocolate is unstable. That’s evident if you keep chocolate for too long or in warm conditions. The chocolate will lose it sheen and get a white film of sugar or fat – a sign that the chocolate is moving towards crystal form six.
Science of steel and chocolate
Making chocolate is therefore purely a matter of materials science. That also makes it an excellent subject for talking about science, especially in the month of December. In December 2018, Marcel Sluiter gave a lecture at the University of Twente in Enschede. He did that together with local chocolatier Jan Meen. ‘The participants were all given a bag of chocolate that they were allowed to taste during the lecture to discover the different phase types,’ Sluiter says.
In March 2017, there was even an entire symposium in Delft where chocolate and steel experts from the four technology universities spoke about the underlying processes and structures of chocolate and steel. ‘We already have a solid understanding of the principles of thermodynamics and the kinetic processes that steel and chocolate share. But that probably wasn’t the case 100 years ago, when the basic principles weren’t as well known yet. The current research focuses on the details of the processes, and in that sense there are differences between steel and chocolate,’ Sietsma says.
Future-proof
That certainly doesn’t mean that there’s nothing left to discover in steel research. One line of research is improving the blast furnace process. ‘A great deal of CO2 is released during the production of steel, especially during the extraction of iron from iron ore. This step is thus extremely important towards making the steel industry more sustainable,’ Sietsma explains. On the other hand, steel is the most reused material in the world: in 2014, 86% of steel waste was recycled. ‘Steel can be endlessly reused, much more so than plastic, for example,’ Sluiter says. Still, every year about 1,800 million tons of steel is still being produced. That’s because we continue to build new cities, bridges and railway lines. ‘But the raw materials for that steel, iron and carbon, are cheap and plentiful. Moreover, it contains few special elements. So steel is a highly future-proof material.’

Marcel Sluiter