Catalysis is the driving force behind the production of fertiliser, which allowed the world population to triple over the past seven decades. And now, it will be just as essential in tackling the global challenge of CO2 emissions. Atsushi Urakawa develops catalysts and catalytic processes to turn this abundant resource into useful chemicals, such as sustainable fuels.
“More than ninety percent of chemicals go through a catalytic process during their production,” says Atsushi Urakawa, professor of catalysis engineering at the department of Chemical Engineering at TU Delft. “Yet, catalysts are like magic stones: we don’t know how they work, but they work beautifully – making chemical reactions run much faster, reducing the energy needed for these reactions and allowing us to steer reactions towards the desired end-products.”
In the 90’s, Urakawa chose to study chemistry because of its practical impact in solving then current problems such as acid rain. He quickly developed a fascination for catalysis, ultimately moving into the field of CO2 chemistry. “Rather than storing CO2 underground, we can use catalysis to convert it into useful chemicals such as fuels,” he says. “The normal trial and error approach to improve these catalytic processes is not bad, but it takes a lot of money and twenty to thirty years’ time to develop. Looking at the timescale of the global challenges we face, we need to accelerate. It is my belief that a rational design of catalysts and the catalytic process is the solution.”
Catalysis
A catalyst is a substance that speeds up a chemical reaction without the substance itself undergoing any change. Catalysts are widely utilised by the chemical industry in processing petroleum and to create all kinds of bulk and fine chemicals. Countless products we use in our daily lives also depend on catalysts: gasoline, fertilisers, and everything that contains plastic, to name a few.
New catalytic materials and processes
Shifting to a rational design is much easier said than done. One of Urakawa’s past supervisors even told him that rational catalyst design was impossible because of the range of timescales and length-scales involved. “Catalysis is a black box at all these scales, but I think it is possible,” Urakawa says. “We just didn’t have the proper tools before to open this black box.”
Most researchers in the catalysis community focus on the atomic scale (nanometre scale) – if the reaction doesn’t work at that scale, it won’t work at the larger scale of a reactor either. Urakawa’s interest, however, is much wider. That is why, after studying chemistry, he travelled the world to also become proficient in quantum chemistry and chemical engineering. “My expertise is looking at catalysis holistically, from the atomic scale up to the reactor scale. What is happening where and what species are present, how are reaction products transported? Without understanding what is happening in the reactor, we can never rationally design a catalytic process."
New catalytic materials and processes
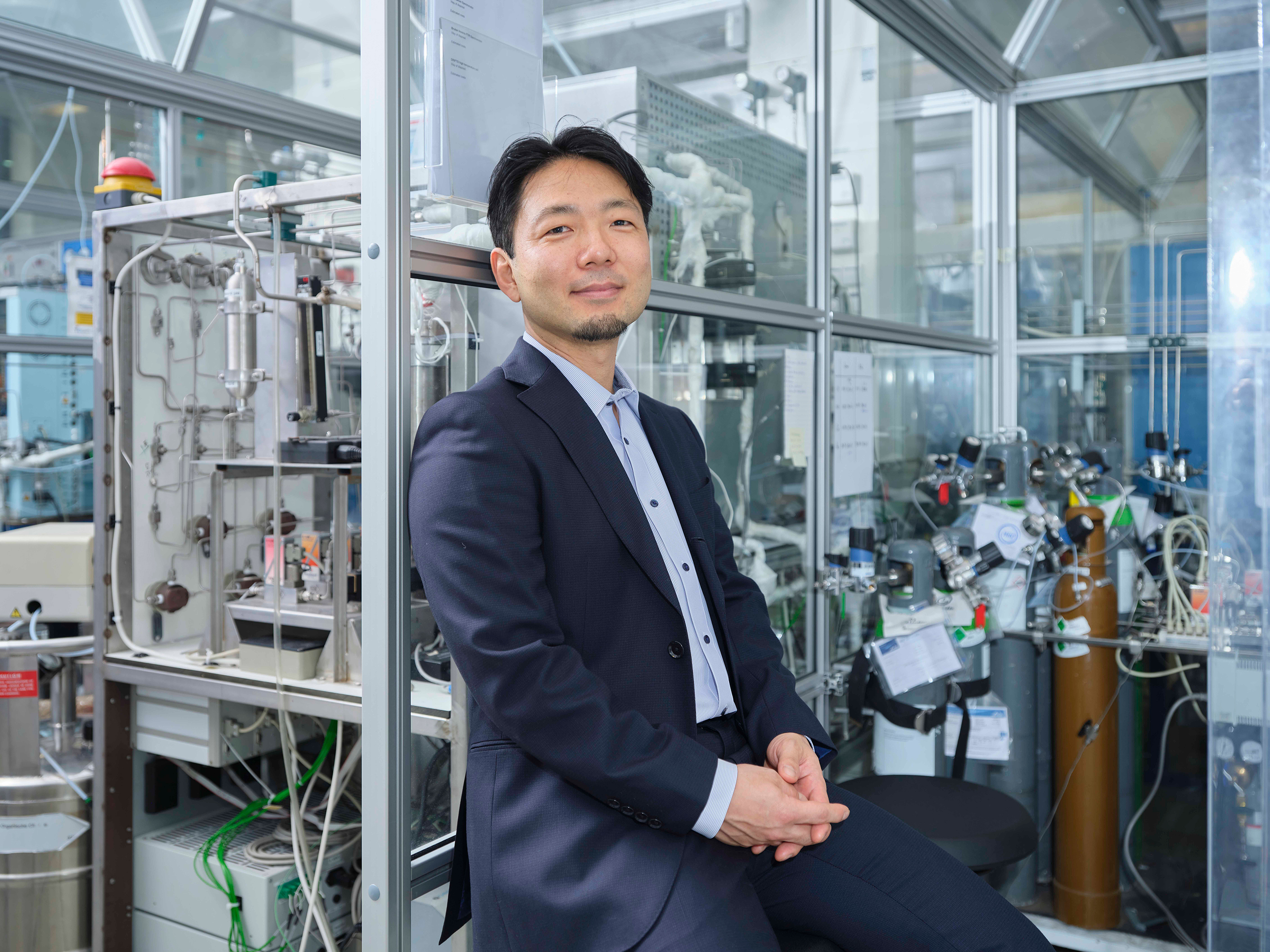
One of the key activities in his research group at TU Delft therefore is so-called operando spectroscopy, which is used to look into the catalyst and the reactor under industrially-relevant operating conditions. Based on that understanding, he develops new catalytic materials and new processes – mainly for the sustainable conversion of CO2 into fuels like methanol, but also for the synthesis of ammonia, and hydrogen.
“These catalytic processes have many challenges in common,” Urakawa says. “We want to lower the amount of energy needed to run these processes and increase their selectivity in creating the desired end-product. Long-term stability of the catalyst is important as well.” His group is unique in that it researches all three catalytic processes: “classical” thermal catalysis at high temperature and pressure, electrocatalysis (using green electricity) and photocatalysis (using light). “We want to know which way is best, and if a combination of methods can be even better.”
Jam-packed with industries
The return to his alma mater TU Delft is instrumental in allowing Urakawa to satisfy his nearly endless curiosity into the working of catalytic processes. “There are so many scientists here covering all scales; molecular physics, thermodynamics, transport phenomena, reactor design, you name it. In my group, we have core expertise for each of these topics. And when we need world-class expertise on any topic, we look for collaborations – within our own department, with other faculties of the TU Delft, or with other universities.”
The favourable industrial climate also played a role in him moving here. “The Netherlands is a small country, but it has a very long and strong history in catalysis and it is jam-packed with chemical industries,” he says. “To achieve our goals, it is crucial to interact with industry on a daily basis.”
(No) end in sight
Another reason for re-joining TU Delft has been his passion for education: Urakawa had been able to pursue an excellent master’s education and wanted to return the favour. Urakawa: “The technical challenges in accelerating catalytic process design are too great for me and my generation alone to tackle. I hope I can be a stepping-stone for those coming after me to continue this work.”
Next to the technical challenge, there is also an economic aspect that hampers large-scale sustainable conversion of CO2 with the use of renewable energy and renewable hydrogen. “Economically speaking, catalysis competes with fossil resources, which are very cheap,” Urakawa says. “But the price of renewable energy is going down, and the price of fossil energy is going up due to carbon taxing. It will become competitive, and we’ll have the proper technology in place when it does.”