DNA origami already is an unrivalled construction method for building nanoscale structures with near-atomic precision. By combining it with machine-inspired design, Sabina Caneva adds mechanical reconfigurability. She uses this to build nano-actuators and size-adjustable nanopores for next level biosensing.
Sabina Caneva earned her nanotechnology stripes working on two-dimensional materials and nanometre-sized pores for biosensing. But a few years ago, she reached the limits regarding the latter. “Solid state nanopores can have arbitrary sizes, but they enlarge over time, making sensing of biomolecules less effective,” she says. “And protein nanopores have exquisitely precise diameters, but these are typically too small to allow the transport of macromolecules, such a potential therapeutics.”
That is when she decided to add a DNA origami research line, as it presented all kinds of solutions. “We wanted to make smart nanopores that not only have well-defined channels but in which the channel size can be controlled on-demand,” she says. “This will make the pores size-selective, which is important in transport and sorting applications. It is impossible to achieve this kind of control and flexibility using solid state materials or proteins.”
DNA origami
Introduced in 2006, DNA origami is a powerful and innovative technique that leverages the unique properties of DNA to create nanoscale structures with remarkable precision. “The fundamentals of how to create pretty much any one-, two-, or three-dimensional shape have long been worked out,” Caneva says. “The recent game changer has been to add specific functionality through the precise attachment of useful components. These can be nanoparticles, fluorescent dyes, enzymes, or antibodies for molecular sensing, to name a few.”
A fresh spin
As an assistant professor in the Precision and Microsystems Engineering department, Caneva has her own group of eight researchers. The novelty of their DNA Origami research is that they create mechanically adaptable structures, such as the pores and actuators mentioned earlier. A variety of mechanical engineering principles is possible, such as hinges, tweezers, and rotors. Inspired by real world mechanical structures, they do behave differently when scaled down to the nano-level and operating in a liquid environment dominated by the random motion of molecules.
It takes a lot of multidisciplinary expertise to ensure both rigidity, mechanical flexibility, and optical functionality of DNA origami
“Since the structures are so tiny, we also want them to emit light of a particular colour when they change shape so we can monitor their mechanics by looking at them under a microscope,” Caneva says. “It takes a lot of multidisciplinary expertise to ensure the required rigidity, mechanical flexibility, and optical functionality.” It is therefore quite intentional that her group has an optical engineer, mechanical engineer, nano-electronics engineer, materials scientist, physicist, and a physical chemist.
“Everybody brings a flavour from their field. We all learn from each other, and I really think it helps to solve the problems we face. Conversely, I think that putting a fresh spin on DNA origami by adding a mechanical engineering component makes my group interesting for researchers to join. It gives them the opportunity to innovate at the intersection of several disciplines and contribute to building something never seen before.”

Cellular communication channels
Thanks to a brilliant postdoc, the size-adjustable nanopores are no longer a dream but a reality. Though still in the proof-of-concept phase, they may eventually be used for size-selective sensing and transport of biomolecules, for instance for targeted drug delivery. “We are currently measuring what can pass through the pores when open, closed or in an intermediate state. We also want to develop strategies for speeding up the shape changes that currently take minutes.”
These types of nanopores are also poised to play a part in the gargantuan effort to build a synthetic cell, which is a major research theme at TU Delft. “Built entirely from the ground up from non-living molecules, the synthetic cell should be able to grow, divide, sense and adapt to its environment – all fundamental characteristics of life,” Caneva says. “The goal for our nanopores is to function as the communication channels in the cell membrane – for letting nutrients in, waste out, and signalling molecules through.”

Low-cost treatment monitoring
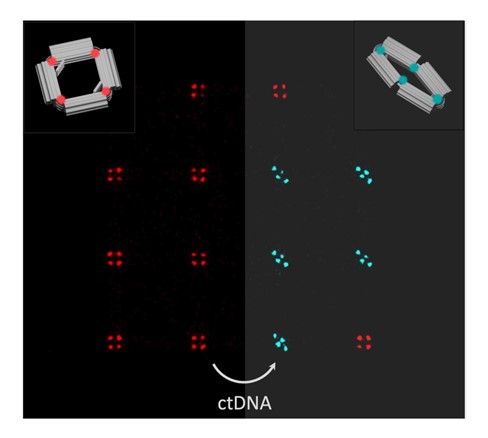
The nano-actuators her group is developing should be able to detect the minute amount of circulating tumour DNA that circulates in the blood stream of a cancer patient. “Current technology for monitoring cancer progression after treatment is much too expensive to allow regular monitoring,” she says. “A medical foundation contacted me to develop a high-throughput, high-sensitivity device that is so cost-effective that it allows monthly screening. With DNA origami you can make a million actuator copies in a single reaction.”
The working principle is for the actuator to change its shape in response to a piece of such DNA binding to it. “We’ll attach fluorescent probes to the origami, allowing us to measure the miniscule shape change with light. We expect that achieving single molecule resolution is within reach.”
The optimal shape of the actuator is work in progress. It could be shaped like a tweezer, an ‘X’, a trapezium, you name it, as long as it is shape-adaptable. Caneva: “That is why I like DNA origami. You are not limited to one structure. With some limitations, you can probably make anything you can imagine.”