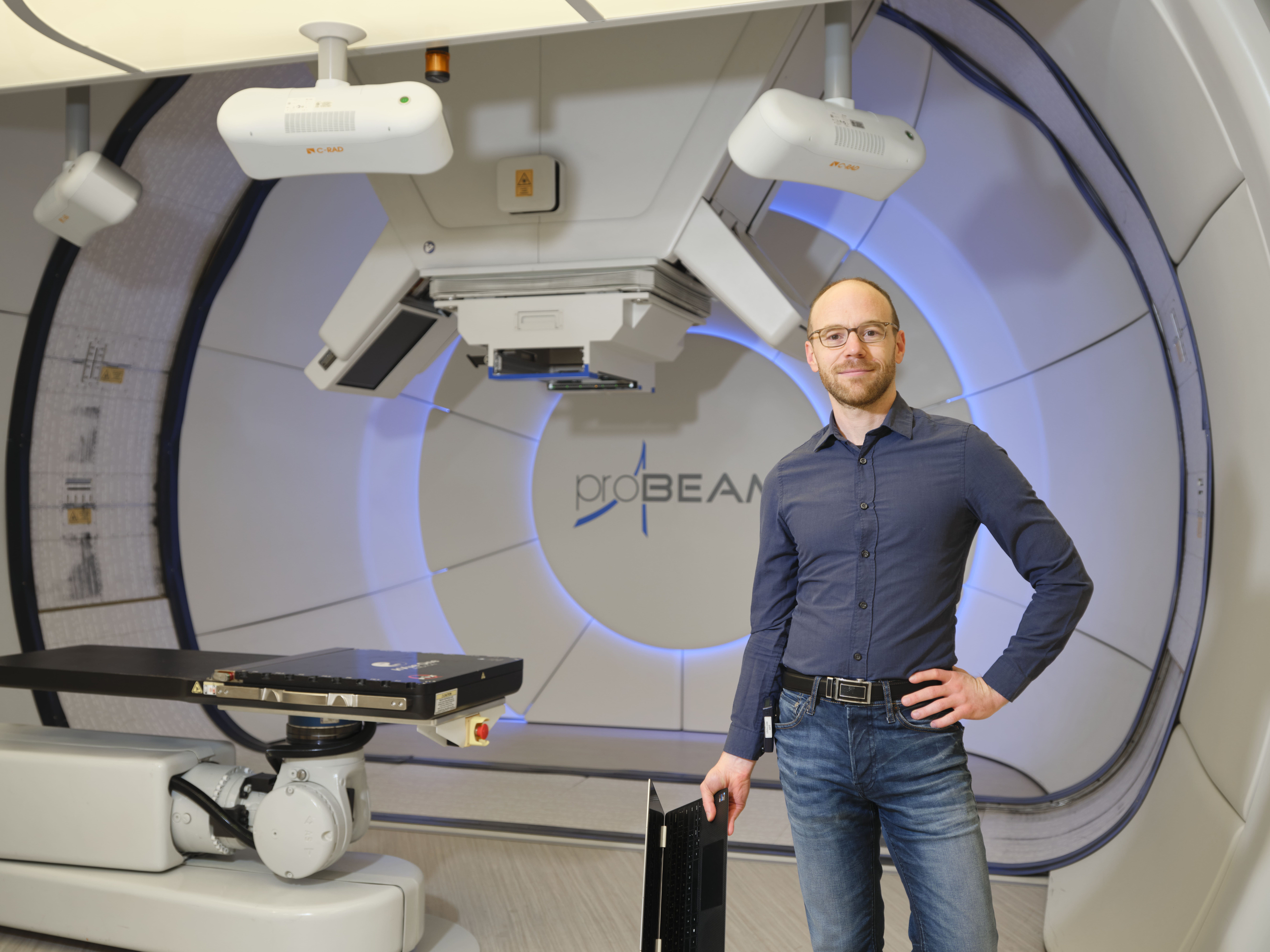
“10 million people a year die from cancer, but many millions survive long term after receiving radiotherapy treatments, so if you can make a computational model that increases effectiveness or decreases side-effects by even just a few per cent, that can help thousands of patients,” says Zoltán Perkó, a specialist in Deep Learning, AI and Computational Physics. Based at TU Delft’s department of Radiation Science and Technology, Zoltán is developing novel modelling tools and numerical methods to make the targeting of tumours for proton therapy much more precise, thereby reducing damage to the surrounding healthy tissues: “The over-arching goal is to give the best possible treatment to every single patient.”
Nuclear reactors
When Zoltán was a young boy, he wanted to be a pianist “and an inventor” so how did he end up leading a team of researchers who are developing the use of Artificial Intelligence (AI) and Deep Learning methods to optimise proton therapy for cancer patients? “It turned out I was better at maths and physics than I was at music,” jokes Zoltán, who studied Engineering Physics and Nuclear Techniques at the Budapest University of Technology and Economics. “I had two fantastic teachers there who got me really interested in reactor physics and the use of simulations to solve fundamental safety issues around nuclear power production in so-called Generation 4 reactors.”
Nuclear power is important in Hungary, providing around 45% of the country’s electricity: “There’s no oil or natural gas, no high mountains for hydropower, so nuclear power is kind of a necessity as there’s not much else,” explains Zoltán. His Master’s research therefore focused on using computational science to investigate how to solve the main challenge of nuclear power: nuclear waste.
As part of his Master’s, Zoltán spent some time at TU Delft, which has a long-established exchange programme with Budapest University: “In those four months, I analysed the fuel cycle of one of the new Generation 4 reactors, so-called gas cooled fast reactors. These reactors are very good at converting long-lived nuclear waste into much less dangerous by-products, so I simulated how we could maximise this transmutation of waste as much as possible.” This was an impressive achievement and upon graduating, Zoltán was immediately offered a PhD position at TU Delft’s Department of Radiation Science and Technology. The goal of his PhD research was to develop algorithms to quantify uncertainty as efficiently as possible.
Uncertainty Quantification Research
So what is uncertainty quantification? “When you have a complex system, say an airplane, a lot of design work goes into building a very detailed computational model, exploring what would happen under certain circumstances, like flying in a storm or landing with strong cross-winds. In order to calculate how these systems would behave in such cases, you need very complex models, and as there are always uncertainties, we need to know how these would impact the performance of the plane. That’s the idea behind uncertainty quantification: recognising that there are all these unknowns, and trying to work out their effects so that the final design is safe and efficient.” In practice this involves running a lot of simulations, which can be very expensive, so part of Zoltán’s research was aimed at trying to speed up this whole process. Four years later, with a PhD in Uncertainty Quantification in the field of nuclear reactor physics, Zoltán made his “big change” to medical physics.
From Nuclear Waste to Proton Therapy
“This was in 2014, when the Netherlands was also beginning to discuss the introduction of Proton Therapy into the medical world as a state-of-the-art form of radiotherapy for cancer patients. The main advantage of Proton Therapy compared to other types of radiotherapy is that it allows you to irradiate the tumour much more accurately without damaging the surrounding healthy tissues. This means the targeting has to be very precise, which was immediately a connection with uncertainties.” In fact it turns out that there are lots of uncertainties connected with patients and their treatment: “For example, a course of radiotherapy is typically spread out over several weeks, and patients can change over that time – they lose weight, maybe the tumour changes shape or size, and of course, every patient is different.”
The main advantage of Proton Therapy for cancer patients is that you can irradiate the tumour much more precisely without irradiating the surrounding tissues.
Radiotherapy Optimisation
Zoltán was offered a position at Harvard University to do his post-doctoral research on radiotherapy optimisation: “Working in the US was always a dream of mine and I loved every minute of it!” One may ask: with a background in nuclear reactor physics, wasn’t jumping into medical physics a bit of a challenge? “Reactors and patients are very different of course but my research was focusing on so-called non-intrusive methods, meaning you don’t need to know the details of the problem as the technique for optimisation of uncertainty quantification is the same. So whether you use my algorithms on a nuclear reactor or a cancer patient, it doesn’t matter – it’s the same technique.”
The Power of Deep Learning
After two years in the States, Zoltán was tempted back to Delft by the offer of a faculty position, and the chance to establish and run his own research group, which is currently developing Deep Learning and AI methods for use in both medical therapy and nuclear reactors. “With computational science, you have basic rules about how the world works, and we put those rules into a computer and then we simulate complex systems that work according to those rules. This means that we can use these simulations to calculate things to help us analyse our system or optimise our design. The huge power of Deep Learning is that it kind of comes from the other direction – it says ‘I don’t know anything about the rules, or how the world works but I have lots of data, so I can try to learn how the world works.’” In practice this means that if we have a lot of digital images, for example from thousands of scans of patients who have tumours, Deep Learning techniques can develop new rules based on what they have learned from analysing all those images to detect where the tumour is and then suggest the best possible treatment.
Working with the Holland Proton Therapy Centre in Delft and the Erasmus Medical Centre in Rotterdam, Zoltán’s group has already contributed to the improvement of treatments. “Our uncertainty quantification methods have helped ensure that proton therapy as a form of cancer treatment is both robust and safe.” In addition, uncertainty research is also helping to correct for the movement caused by patient breathing – an important challenge when trying to target a moving tumour with the highest precision. “We’ve developed the current state-of-the-art in fast and accurate dose calculations via a Deep Learning method which means that in the future, we could possibly change dosages from day to day as necessary, or even in real time.”
Making impact
Although Zoltán is also still working in the field of nuclear safety, he’s extremely happy with his choice to go down the medical physics route: “First of all I just love the field – the medical field is much more impactful in a sense… I mean what I was doing with the nuclear energy research was about new generation nuclear reactors, and there are zero existing today, whereas there are around 100 proton clinics in the world already operating and treating patients: so whatever you can do there has a much more straightforward route towards actual application. I like the nuclear field, and there’s wonderful, crucially important research to do there, but in clinical practice, we can make improvements right now! So for me that’s a big part of the motivation for sure.”