The 1st Electrochemical Conversion National Symposium
The future of Electrochemical Conversion
- Hydrogen and beyond -
Exploring the Future of Sustainable Chemicals and Fuels through Electrochemistry
The 1st National Symposium on Electrochemical Conversion took place on May 21st in the Netherlands. This event was a great success, below you find an overview and highlights of the day's activities.
The symposium focused on exploring the potential of electrochemistry to drive the development of sustainable chemicals and fuels. Experts from across the field gathered to discuss the latest advancements and future prospects in this important area of research and innovation.
Overall, the 1st National Symposium on Electrochemical Conversion was a success.

On behalf of the entire organizing committee, I would like to thank all of the speakers, poster presenters and attendees for making the ECCNS symposium a great success. We hope that you were inspired by all the great work on electrochemical conversions that is carried out by our community and made new connections that will help in future endeavours.
Personally, I was amazed by the high quality of presentations and posters by junior scientists, convincing me that we as a Dutch community can make a difference in solving the challenges related to employing electrochemical technologies in the near future to produce chemicals and fuels. I hope you thoroughly enjoyed your day at the ECCNS and hope to see you during the next edition of this symposium!
Ruud Kortlever
Associate professor at TU Delft
Opening plenary session
“We’re trying to convert CO2 into products, but it’s very tough to compete with hydrogen. Our focus point should however not be the cost per ton of product, but the value per used energy unit. This means we can be less efficient and still obtain a higher value per used electron.
Our group studies salt formation and movement of ions in CO2 electrolyzers, so we understand what needs improvement. As it is right now, we still need 67% of Europe’s energy consumption to fulfil the world’s demand of only ethylene. Improving efficiency and stability of copper-based CO2 electrolysis is key in scaling this process."
Morning breakout sessions
(Bio) CO2 electrochemical conversions - electrolyser
“We need to use CO2 to close the carbon cycle but, as it is, we’re still 8 to 9 orders of magnitude away from the scale we need for that. In scaling these devices, we need to supress loss and crossover of CO2, otherwise the energy consumption gets so large that the electrolyzers don’t make sense. This is where, I think, engineering liquid-liquid electrolyzers still holds promise as a solution.” David Vermaas took us through his lab’s advances in CO2 electrolysis.
Afternoon plenary session
“In a sense, what everyone here today is trying to do is revolutionary. We’re trying to reverse one of the most famous reactions in the planet, the combustion of hydrocarbons, by just using CO2, water and electrons. And we need that, because the vast majority of our chemicals industry still has a fossil origin.
“In developing and studying these solutions, we need to keep a systems perspective in our work. For example, comparing our findings to traditional systems makes little sense. As of now, for example, CO2RR is 30x as intensive as direct-air capture of CO2. But, in our modelling we’ve seen already feasibility of integrated recycling streams. We’ve seen a lot of fancy numbers and performance metrics, but they’re irrelevant if we do not consider the impact on the wider value chain. A systems analysis helps us to find the best trade-offs in system design and engineering”.
Afternoon breakout sessions
(Bio) CO2 electrochemical conversions - component
“As of now we’re developing electrolyzers mostly as black boxes. Using a variety of techniques, our group can keep an open perspective and analyse all the phenomena happening at a very small scale in our electrolyzers.
“For example, using in-situ Raman, we can follow the formation of intermediates on copper for the CO2 to hydrocarbons process. Similarly, using fluorescence and specially designed particles, we can probe the temperature of the interface while applying a current, which tells us more about real-life conditions.” Ward van der Stam took us through his group’s efforts at Utrecht University in the afternoon breakout sessions.
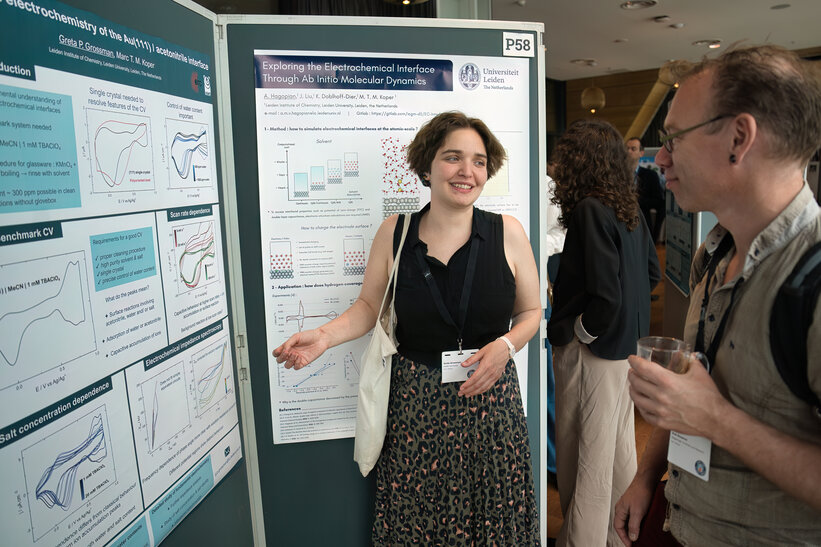
Poster awards
To close off the day, the poster awards sponsored by GroenvermogenNL were announced by Paulien Herder, chair of GroenvermogenNL. The third prize was a check for EUR 250, the second prize EUR 500 and the first prize a check for EUR 750.
After a very pressing evaluation period, the jury has come to the following decision:
1st price - Hugo-Pieter Iglesias van Montfort: Current distribution on flooding resistant electrodes for CO2 reduction
Very clear step by step conclusions that were made with self-explanatory images. An original concept potentially applicable to several fields with a variety of proof of concepts. The jury liked the descriptive titles that allow for limited reading to understand the primary point of each section. The graphic generated imagery of the work conveyed the geometries tested which allowed readers to understand the results much faster and clearer.
2nd price - Zhiyu Zhu: Operando NMR methods for studying electrochemical CO2 reduction
The use of a benchtop NMR for in-situ product detection is a very unique application with good preliminary results with time and potential resolution. Secondary measurements such as pH and formate add an additional research perspective. Overall the concept of benchtop NMR’s have potential to extend to other electrochemical applications, particularly as more complex reaction products maybe only detectable through liquid-phase analysis.
3rd price - Vojtech Konderla: Seawater CO2 capture using bipolar membrane electrodialysis
The approach is uniquely paired to existing applications of desalination such that the feedstock is more ion dense. The work then clearly recognises and discusses the challenges that this faces due to added scaling and its penalty on performance. In the end the results are put into a greater context. The judges liked the clear section headers and progress structure.
In the coming years the ECCNS symposium will continue
The Electrochemical Conversion National Symposium (ECCNS2024) brings those interested in green chemistry and green hydrogen together, in order to share the very latest insights in science and research.
I am convinced by sharing knowledge as quickly and as openly as possible, the Netherlands can really accelerate the transition to a green society. This sharing takes place between scientists, but also between scientists and companies.
Knowledge exchange in both directions provides mutual inspiration. GroenvermogenNL facilitates and accelerates this interaction: we bring companies in direct contact with scientists and we ensure that scientists can in turn learn from the findings in practice.
In the coming years, we will continue to cooperate with ECCNS, this year in collaboration with TU Delft and in the future with at least one of the universities in the Netherlands. That way, we can effectively use the funds allocated to GroenvermogenNL by the National Growth Fund to make the Netherlands' innovation engine run even faster. With this innovation, we create new jobs, and a new sustainable economy.
GroenvermogenNL is proud to be a sponsor and co-organiser of this symposium.
Paulien Herder
Dean TNW faculty at TU Delft | Board member GroenvermogenNL
09:00 - 09:30 | Registration (Wardrobe) + Coffee (Foyer 2) |
| |
Plenary session | |
09:30 - 10:15 | Plenary speaker Brian Seger | A synchrotron analysis of high current density CO2 electrolysis devices |
| |
Breakout session 1: Water electrolysis | |
Amare studio | Chair: Fokko Mulder |
10:30 - 11:00 | Keynote Thijs de Groot | Increasing performance, flexibility and durability of Alkaline Water Electrolysis |
11:00 - 11:15 | a | Onno van der Heijden | Li+ Cations Activate NiFeOOH for Oxygen Evolution in Sodium and Potassium Hydroxide |
11:15 - 11:30 | b | Sohan Phadke | Modelling Anodic H2O2 Accumulation in Alkaline Water Electrolysis with Experimental Validation |
11-30 - 11:45 | c | Venkataramana Rishikesan | Catholyte-free Anion Exchange Membrane water electrolysis enabled by thin form factor Nanomesh Electrodes |
11:45 - 12:00 | d | Maximilian Demnitz | Catalyst coating of diaphragms for increased performance in alkaline water electrolysers |
| |
Breakout session 2: (Bio) CO2 electrochemical conversions - electrolyser | |
Room: Swing | Chair: Tom Burdyny |
10:30 - 11:00 | Keynote David Vermaas | Scalable cell architectures for CO2 electroconversion |
11:00 - 11:15 | a | Merin Varghese | The effect of electrolyte flow rate on product selectivity in CO2/CO electrolysis |
11:15 - 11:30 | b | Siddhartha Subramanian | Enhancing hydrocarbon production in a copper-based zero-gap carbon dioxide electrolyzer through spatial modulation of CO2 and CO |
11-30 - 11:45 | c | Hugo-Pieter Iglesias van Montfort | Mapping Spatial Effects in CO2 Electrolyzers |
11:45 - 12:00 | d | Mu Lin | Low energy consumption AEM-based electrochemical carbon capture process design |
| |
Breakout session 3: Electrochemical production of NH3, C-N and specialty chemicals – Session 1 | |
Room: Jazz 3 | Chair: Ruud van Ommen |
10:30 - 11:00 | Keynote Marta Costa Figueiredo | Electrocatalytic synthesis of fertilizers |
11:00 - 11:15 | a | Min Li | Polymer Modified Ru Nanoparticles Boost Electroreduction Nitrate to Ammonia in Water-fed PEM Cell |
11:15 - 11:30 | b | Boaz Izelaar | Electrochemical Ammonia Synthesis via Lithium-mediated Nitrogen Reduction |
11-30 - 11:45 | c | Ruipeng Luo | Operando NMR study of electrochemical lithium-mediated ammonia synthesis |
11:45 - 12:00 | d | Phebe van Langevelde | The Electrochemical Production of Hydrogen Peroxide with Molecular Copper Catalysts |
| |
Breakout session 4: Integrated equipment & Plant design | |
Room: Jazz 4 | Chair: Ana Somoza-Tornos |
10:30 - 11:00 | Keynote Mar Pérez-Fortes | Exploring the implementation of CO2 electrolysis for syngas production: scaling strategies and trade-offs |
11:00 - 11:15 | a | Jeroen Homan | Processing of PEM fuel cell catalyst layers |
11:15 - 11:30 | b | Akmal Irfan Majid | A Rotating Disc Electrochemical Reactor to Produce Iron Powder for the CO2-Free Iron Fuel Cycle |
11-30 - 11:45 | c | Esteban Camilo Lage Cano | A two-stage optimization approach for the design of an electrochemical plant powered by solar energy |
11:45 - 12:00 | d | Josephine Vos | Ex-ante techno-economic assessment of a co-electrolysis-based plant for synthetic fuel production |
| |
12:00 - 13:30 | Lunch + Posters (Foyer 2) |
| |
Plenary session | |
13:30 - 14:15 | Plenary speaker Andre Bardow | Power to the plastics! A systems perspective on a sustainable chemical industry |
| |
Breakout session 5: Scaling, dynamic operation & grid connection | |
Room: Jazz 3 | Chair: Mar Pérez-Fortes |
14:30 - 15:00 | Keynote Matteo Gazzani | Green Hydrogen: From Production to Industry Applications and Technological Advancements - Insights from energy system modelling |
15:00 - 15:15 | a | Lucas Cammann | A plantwide control structure for improved flexible production of green hydrogen |
15:15 - 15:30 | b | Jan Wiegner | Unleashing the full potential of the North Sea – The role of hydrogen towards 2030 and beyond |
15-30 - 15:45 | c | Thijmen Wiltink | Optimal CO2-based syngas supply chain configurations: Insights into location and scaling |
15:45 - 16:00 | d | Özlem Mahmutogullari | Sustainable supply chain design for CO2 electrolysis under CO2-based syngas demand uncertainty |
| |
Breakout session 6: (Bio) CO2 electrochemical conversions - component | |
Amare Studio | Chair: Ruud Kortlever |
14:30 - 15:00 | Keynote Ward van der Stam | Probing the dynamics of CO2 electrolysis with X-ray and Raman scattering |
15:00 - 15:15 | a | Rafael Vos | How Temperature and Pressure Affect the Selectivity of the Electrochemical Reduction of CO2 at Au, Cu and Ni |
15:15 - 15:30 | b | Matt Peerlings | Improving durability of Cu-based electrodes for CO2 reduction |
15-30 - 15:45 | c | Shilong Fu | Electrochemical CO2 Reduction in the Presence of SO2 Impurities on a Nitrogen-doped Carbon Electrocatalyst |
15:45 - 16:00 | d | Senan Amireh | Engineering gas diffusion electrode microstructures for the electrochemical reduction of CO2 |
| |
Breakout session 7: Electrochemical production of NH3, C-N and specialty chemicals – Session 2 | |
Room: Swing | Chair: Marta Costa Figueiredo |
14:30 - 15:00 | Keynote Ludovic Jourdin | Microbial Electrosynthesis from CO2 reaches Productivity of Syngas and Chain Elongation Fermentations |
15:00 - 15:15 | a | Pim Broersen | Electrochemical glycine production from biomass derived starting chemical materials |
15:15 - 15:30 | b | Talal Ashraf | Electrooxidation of Carboxylic Acids on Boron Doped Electrodes for Pyrolysis Oil Upgradation Application |
15-30 - 15:45 | c | Ivo van Luijk | Enhancing Faradaic Efficiency in Electrocatalytic H2O2 Production: Geometrical Particle Size Effects of Non-Noble Transition Metal Nanoparticles |
15:45 - 16:00 | d | Adam Vass | Electrochemical formation of H2O2 on a boron-doped diamond-coated mesh anode in a zero-gap PEM electrolyzer |
| |
Breakout session 8: Integrating electrolysis with current processes and systems | |
Room: Jazz 4 | Chair: Atsushi Urakawa |
14:30 - 15:00 | Keynote Melis Duyar | Designing dual function materials for integrated carbon dioxide capture and utilisation |
15:00 - 15:15 | a | Eliana Lozano | Integration of BtL and PtX for the production of renewable fuels and ethylene |
15:15 - 15:30 | b | Julia Tiggeloven | Exploring the role of electrolyzers in the decarbonization of the ammonia industry |
15-30 - 15:45 | c | James Tonny Manalal | The relevance of hydrogen origin for the selection of alternative carbon based technologies - A case study of alternative ethylene production pathways |
15:45 - 16:00 | d | Sanghamitra Chakravarty | Exploring enablers and barriers shaping carbon dioxide electrolysis in Europe: A policy and governance perspective |
| |
16:00 - 16:30 | Closing + Poster prize (Amare Studio) |
16:30 - 17:30 | Borrel (Foyer 2) |
17:30 - 20:00 | Dinner (Foyer 3) |
Keynote Speakers
Plenary Speakers